1. Background
Antibiotic resistance, commonly known as antimicrobial resistance (AMR), is a critical public health concern and poses a significant challenge for the prevention and treatment of chronic medical conditions. This phenomenon involves the development of resistance by microbes to antibiotics that were previously effective in treating infections. The consequences of AMR are severe, leading to increased morbidity, mortality, and healthcare costs, as well as reduced efficacy of medical interventions. Addressing this issue requires a comprehensive approach that includes the development of new antibiotics, the rational use of existing antibiotics, and the implementation of effective infection prevention and control measures. Failure to address AMR could have catastrophic consequences for global health and necessitates urgent action by policymakers, healthcare professionals, and researchers. Despite various measures taken in recent decades, there is no sign of a global slowdown in AMR (1). Antimicrobial resistance occurs when bacteria continue to thrive and propagate despite efforts to treat them with medication, finding ways to defend against antibiotics (2). The European AMR Surveillance System (EARSS) has monitored the effectiveness of medications against bacteria in Europe since 1998. According to their research, resistance to antibiotics in Europe correlates with the use of certain types of antibiotics, such as beta-lactams and macrolides (3).
Food spoilage also presents significant challenges for the food industry, leading to considerable increases in food waste and imposing financial burdens on food producers and consumers. This issue can damage a brand’s reputation. Food can become infected by fungi at multiple stages, including after harvesting, during processing, and storage. The growth of fungi can alter the flavor, appearance, or scent of food. Some molds produce toxins that can cause illness in humans. Rhizopus stolonifer is a common and rapidly growing fungus belonging to the Zygomycota group. This fungus causes diseases known as soft rot, black mold, and Rhizopus rot. It can thrive quickly in wet and humid environments due to the prevalence of its airborne spores. R. stolonifer is responsible for the spoilage of many fruits and vegetables post-harvest (4).
Penicillium is a saprophytic fungus that contaminates food, fruits, and soft drinks, leading to clinical contamination (5). Cladosporium fungi are found in various regions globally, with their spores present in the air, soil, and water (6). They are commonly isolated from residential and public areas, as well as food products (7, 8). These fungi require cool and moist conditions to thrive, spread, and grow, and they are best suited to cool and damp environments (9). Dermatophytes are a group of keratin-loving fungi that belong to the genera Epidermophyton, Microsporum, and Trichophyton, commonly causing conditions such as athlete's foot and ringworm of the trunk and skin (10). Trichophyton mentagrophytes and T. rubrum are reported as the primary causes of athlete's foot worldwide (11).
Food companies are increasingly looking to reduce their reliance on chemicals and explore natural methods for preserving food (12). Microbial resistance and food spoilage are growing concerns. Fungi resistant to drugs and some food preservatives have become major issues related to human poisoning and food spoilage, presenting serious challenges to the pharmaceutical and food industries. Thus, discovering new methods and materials to address these issues is essential. Historically, people have utilized the natural resources around their homes for food and medicinal purposes. Over time, they have learned to use plants for both sustenance and healing by experimenting with various methods (12). The use of plants for medicinal reasons is a longstanding practice, as these plants contain important elements that can help fight off infections and diseases. These compounds are primarily found in plants, though they can also be sourced from the microorganisms that inhabit them (13).
Phytotherapy, often referred to as herbalism in Western medicine, involves using plants for therapeutic purposes (14). A variety of new natural products with antimicrobial properties have been discovered in plants. Searching for potent antimicrobial chemicals in plants is an effective way to find new medications that can combat the microbes becoming resistant to current drugs (15).
Bacterial endophytes reside inside plants during certain stages of their life cycle and are commonly present in almost all plant species globally. Several research studies have shown that endophytes can be utilized to develop innovative and beneficial products for healthcare, agriculture, and commerce (15). Living within the host plant, endophytes can impact its essential functions. They can promote plant growth, protect it from disease, and help it adapt to challenging environments (16, 17). The genus Allium includes valuable species such as onions and garlic. Allium jesdianum, locally known as Boser, Sarpa, or Bon-Sorkh (due to the red base of the leaves), is a bulbous and perennial plant with 2 to 3 leaves that grows in the western and southwestern highlands of Iran. This plant is harvested by local people in early spring and sold in markets. Allium jesdianum has various nutritional uses and is traditionally used to treat and reduce rheumatic and gastrointestinal pain and facilitate the excretion of kidney stones. Bon-Sorkh soup is considered very effective for treating colds (18). In laboratory experiments, certain types of Allium plants have been shown to reduce the risk of high cholesterol and high blood pressure (19, 20)
2. Objectives
This study evaluated the isolation and characterization of bacterial endophytes from A. jesdianum and their antifungal effects on R. stolonifer, Penicillium, Cladosporium, and T. mentagrophytes.
3. Methods
3.1. Fungal Strains
Four different types of fungi, namely R. stolonifer, Penicillium, Cladosporium, and T. mentagrophytes, were obtained from the Department of Mycology at Tehran University, Iran. These fungi were subsequently recultured in Sabouraud dextrose agar medium, which was procured from Merck, Germany.
3.2. Confirmation of the Plant in Terms of Taxonomy
In the context of a clinical trial study aimed at investigating the antifungal effects of bacterial endophytes, a maximum of 25 fresh samples of A. jesdianum were collected from different regions of Chaharmahal and Bakhtiari provinces, Iran, in the spring of 2023. The taxonomical classification of this plant species was confirmed by the Department of Botany through comprehensive analysis of its morphological and anatomical features. The samples were then transferred to the microbiology laboratory at Shahrekord, Chaharmahal and Bakhtiari University for further investigation. The plant underwent a rigorous preparatory process to ensure it was free of contaminants and ready for further analysis.
3.3. Extraction of Bacterial Endophytes
3.3.1. Disinfection of Allium jesdianum
The sterilization steps for A. jesdianum were conducted sequentially as follows: (1) Different parts of the plant were cut into pieces using a sterile scalpel and exposed to 70% ethanol for 2 minutes before being washed with sterile distilled water; (2) The plant parts were then immersed in a 5.3% sodium hypochlorite solution for 5 minutes and subsequently washed again with sterile distilled water; (3) The plant parts were placed in 75% ethanol for 30 seconds and finally rinsed with sterile distilled water.
These steps ensured that the plant specimens were in optimal condition for further analysis and to maintain the integrity of the study (21). The final rinse water was used as a control and cultured on nutrient agar to verify that it was free of any contaminants. After sterilization, the sections were transferred to agar media for cultivation. Yeast Extract Agar (Merck, 64271, Germany) and peptone agar medium are commonly used in the isolation of endophytic bacteria due to their ability to create a favorable environment for bacterial growth (22). As the samples were cultured, all culture media and control plates were incubated for 4 - 7 days at a temperature of 35°C (22), allowing the endophytes to develop and multiply on the plates. After incubation, the endophytes were randomly labeled for easier identification. Each colony was then macroscopically examined individually to further improve identification (23, 24).
This detailed procedure was essential for the effective isolation of the endophytic bacteria. The use of different types of agar media ensured that the bacteria had the necessary nutrients to grow and multiply. The incubation period was carefully selected to provide sufficient time for the endophytes to grow and develop. The macroscopic examination of each colony led to the accurate identification of each bacterial strain (22, 23).
The endophytic bacterial colonies were purified and studied, with a focus on their morphology, including shape, size, and color. Identification of the bacteria was performed through catalase and oxidase tests, Gram staining, and biochemical assays. These tests were conducted to determine the biochemical properties of the bacteria and their Gram characteristics. The collected data was analyzed to identify the types of endophytic bacteria present in the sample.
3.4. Identification of Endophytes
3.4.1. 16S rRNA Gene Amplification and Sequencing
Genomic DNA extraction was performed using the PGA Bacterial DNA Extraction kit, following the manufacturer's guidelines provided by POUYA GENE AZMA Ltd, Tehran, Iran. A 1500 bp region of the 16S rRNA gene was amplified using universal eubacterial primers F-D1 5'-CCGAATTCGTCGACAACAGAGTTTGATCCTGGCTCAG-3' and R-D1 5'-CCCGGGATCCAAGCTTAAGGAGGTGATCCAGCC-3' (25), utilizing a thermal cycler. The products of amplification were resolved through 1.5% agarose gel electrophoresis and visualized using a gel documentation system, Alfa Imager, developed by Alfa Innotech Corporation, USA. The amplicons were then purified using the PGA Gel purification kit offered by Pouya Gene Azma Ltd, Tehran, Iran, and the quantity of DNA was determined at 260 nm using a spectrophotometer, with calf thymus DNA serving as a control. The purified partial 16S rDNA amplicons were subsequently sequenced.
3.5. Analysis of 16S rDNA Sequences
In this study, a comprehensive analysis of nucleotide sequences was conducted by comparing them to the available sequences in two databases, namely the National Center for Biotechnology Information (NCBI) and EzBioCloud databases. To identify sequences that exhibited high similarity, the Nucleotide Basic Local Alignment Search Tool (BLAST N) program was utilized, available on both the NCBI BLAST server (www.ncbi.nlm.nih.gov/BLAST) and the EzBioCloud server (https://www.ezbiocloud.net). Our criteria for selecting sequences required a minimum of 99% similarity to our partial nucleotide sequences. This approach enabled us to retrieve sequences that were most closely related to our data, allowing us to derive meaningful conclusions from our study.
3.6. Antagonistic Test
Next to each purified and grown endophyte colony with a diameter of 5 mm, the tested fungal samples such as R. stolonifer, Penicillium, Cladosporium, and T. mentagrophytes were cultured and incubated. The Petri dish, with a width of 9 cm and a volume of 10 mL, was maintained at a temperature of 37 ± 1°C for 72 hours. The measurement of potential interactions demonstrated the extent of the antagonistic activity. The culture medium was evaluated for its ability to inhibit the growth of the tested fungi (26).
4. Results
4.1. Physio-biochemical Characterization
This study focused on the collection and analysis of 10 distinct bacterial endophytes from various parts of A. jesdianum. Out of these 10 endophytes, four were classified as Gram-positive, and the remaining six were Gram-negative. Additionally, six of these endophytes were cocci, two were bacilli, and two were cocci-bacilli. This finding is consistent with existing research suggesting that most bacterial endophytes from plants are classified as cocci and bacilli. These endophytes were cultured using various media, including yeast extract agar, peptone agar, blood agar, TSB, Luria Bertani broth (LB), and Mannitol salt agar. The colonies of bacterial endophytes on peptone agar medium showed superior growth compared to those on yeast extract agar (YEA). The formulations for both culture mediums are provided in Table 1. Detailed information on the biochemical characteristics of these endophytes is presented in Table 2.
| Culture Mediums | Abbreviations | Construction Formula |
|---|---|---|
| Yeast extract agar | YEA | Yeast extract 5 g/L (Merck, 64271, Germany) + glucose 10 g/L + agar 16 g/L (Quelab, 420223) |
| Peptone agar | PA | Pepton water 15 g/L (Difco, 1807 – 17 - 4) + agar 16 g/L (Quelab, 420223) |
| Variables | Staphylococcus warneri AJB1 | Staphylococcus succinus AJB2 | Pseudomonas thivervalensis AJB3 | Bacillus subtilis AJB4 | Acinetobacter lwoffii AJB5 | Pantoea brenneri AJB6 |
|---|---|---|---|---|---|---|
| Gram staining | + | + | - | + | - | - |
| Shape | Cocci | Cocci | Bacilli | Bacilli | Cocci | Coccobacilli |
| Catalase | + | + | + | + | + | + |
| Oxidase | - | - | + | + | + | - |
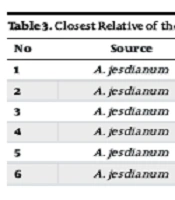
Based on 16S rRNA gene sequencing, the isolates were identified as Staphylococcus warneri AJB1 (Mt642942.1), Staphylococcus succinus AJB2 (MN826566.1), Pseudomonas thivervalensis AJB3 (MK267298.1), Bacillus subtilis AJB4 (QQ566832.1), Acinetobacter lwoffii AJB5 (MW703148.1), and Pantoea brenneri AJB6 (OM095348.1). Table 3 provides comprehensive information about the strains and their closest relatives, determined through 16S rRNA gene sequence analysis.
| No | Source | Strains | Accession Number | Nearest Phylogenetic Neighbor | Similarity, % |
|---|---|---|---|---|---|
| 1 | Allium jesdianum | AJB1 | MT38517.1 | Staphylococcus warneri J20M6LARS | 100 |
| 2 | Allium jesdianum | AJB2 | MN826566.1 | Staphylococcus succinus cqsM8 | 100 |
| 3 | Allium jesdianum | AJB3 | MK267298.1 | Pseudomonas thivervalensis CPs4 | 99/80 |
| 4 | Allium jesdianum | AJB4 | QQ566832.1 | Bacillus subtilis SAB10 | 100 |
| 5 | Allium jesdianum | AJB5 | MW703148.1 | Acinetobacter lwoffii MNA11 | 100 |
| 6 | Allium jesdianum | AJB6 | OM095348.1 | Pantoea brenneri Ais246 | 95.42 |
4.2. Antifungal Activity
The findings, as indicated in Table 4, show that among the 6 tested endophytes, the culture mediums were evaluated for their ability to inhibit the growth of the tested fungi. AJB1 and AJB3 do not display any antagonistic activity, while AJB2, AJB5, and AJB6 exhibit antagonistic action against R. stolonifer, as evidenced by the absence of growth in the culture medium after the incubation period. Furthermore, only AJB4 demonstrates strong antagonistic activity against Penicillium. Except for AJB5, none of the other isolated endophytes show any antagonistic activity against Cladosporium. Additionally, none of the endophytes exhibit antagonistic activity against T. mentagrophytes.
| Isolates | R. stolonifer | Penicillium | Cladosporium | Trichophyton mentagrophytes |
|---|---|---|---|---|
| AJB1 | - | - | - | - |
| AJB2 | + | - | - | - |
| AJB3 | - | - | - | - |
| AJB4 | - | ++ | - | - |
| AJB5 | + | - | + | - |
| AJB6 | + | - | - | - |
Abbreviations: –, no antagonistic action; +, antagonistic action; ++, strong antagonistic action.
5. Discussion
Based on the isolates identified from A. jesdianum, the endophytes of this plant belong to six different species: S. warneri (AJB1), S. succinus (AJB2), P. thivervalensis (AJB3), B. subtilis (AJB4), A. lwoffii (AJB5), and P. brenneri (AJB6). Endophytic bacteria are found in many different plants, including numerous medicinal plants that host these bacteria (27, 28). Several studies have aimed to identify and isolate endophytes from a wide range of medicinal plants. The discovery of endophytic microbes from medicinal plants is considered promising for obtaining antifungal metabolites useful in treating invasive fungal infections. Despite the vast number of medicinal plants presumed to possess antifungal activity, only a few have been explored for endophytic microbes. This study was designed to isolate bacterial endophytes and investigate their antifungal properties. Some endophytes, such as Bacillus spp. and Pseudomonas spp., have demonstrated antifungal and antimicrobial capabilities in plants like tomatoes and ginger (29, 30). Bacillus cereus has been isolated from Chenopodium majus (31), P. putida from Zingiberofficinale (29) and poplar trees (32). Strains of C. michiganensis have been reported as pathogenic to plants like green wilt and corn, but non-pathogenic to prairie plants (33). Allium plants contain important components such as carbohydrates, flavonoids, and saponins, which play a significant role in medicine by providing antifungal, antibacterial, and antitumor activities, as well as reducing inflammation, blood clots, and cholesterol levels (34, 35). Bacteria secrete compounds that can eliminate or inhibit the growth of harmful microorganisms such as bacteria, fungi, viruses, and protozoa, which can cause diseases in humans and animals (36).
Recent research has shown increasing interest in the antimicrobial effects of bacterial endophytes. Notable studies include Kumar et al. in 2016 (37) isolating endophytes from Curcuma longa L., Jiang et al. in 2018 (38), Kumar et al. in 2015 (25), among others. In our research, the AJB2, AJB5, AJB4, and AJB6 species exhibited antifungal properties against R. stolonifer, Penicillium, Cladosporium, and T. mentagrophytes, suggesting that some endophytes are effective in inhibiting the growth of phytopathogenic fungi. The ongoing search for endophytic sources in clinical medicine continues as these organisms produce inhibitory compounds with potential applications in treating fungal and bacterial diseases. Studies have confirmed that endophytes produce novel secondary metabolites as a resistance mechanism to counter pathogen invasion (15).
Endophyte microbial strains are considered antagonistic agents capable of inhibiting pathogens through a non-toxic approach with low side effects. They have shown promising effectiveness against a wide range of pathogens (39). By alleviating the financial and time constraints of upcoming projects, it is expected that the potential for extracting extensive and specific endophytes will increase. To date, research on A. jesdianum is very limited. Most studies have focused on the extract of this plant and its antagonistic activity (40, 41), leaving research on the endophytes of this plant relatively scarce. This gap highlights the significance and potential impact of upcoming studies in this area. The current study is among the few in this field that focus on the isolation of A. jesdianum bacterial endophytes and their observed antagonistic activity against the aforementioned fungi. With the removal of existing financial and time constraints, it is anticipated that a broader range of endophytes could be isolated, potentially leading to more effective antifungal or antibacterial activities.
5.1. Conclusions
However, further research is necessary to fully determine the potential of these endophytes for antibiotic purposes and food applications. The findings suggest the possibility of utilizing these endophytes as natural alternatives to synthetic chemicals in protecting crops against phytopathogens.