1. Introduction
Type 1 diabetes (T1D) is a complex chronic disorder resulting from immune-mediated destruction of insulin-producing pancreatic β-cells. It is often accompanied by various comorbidities such as diabetic ketoacidosis (DKA), long-term complications, and an increased risk of infections (1). The presence of hyperglycemia, due to insufficient insulin production, acts as a double-edged sword that can impair cytokine production in response to infections, reduce chemotaxis and phagocytic activity, and enhance pathogenesis, especially during viral infections (2).
With the initial outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is known as COVID-19, clinical reports have indicated that patients with T1D are particularly vulnerable to severe courses of the infection (3). The hyperinflammatory responses commonly seen in severe cases of COVID-19 can reduce tissue sensitivity to insulin. Additionally, anti-inflammatory therapies, which often involve steroids, are known to increase peripheral insulin resistance (4).
Metformin, a biguanide antihyperglycemic agent, has been shown to improve peripheral insulin sensitivity, reduce hepatic gluconeogenesis, and decrease intestinal glucose absorption. It has also been found to improve outcomes in infectious diseases. Metformin decreases the pro-inflammatory response, enhances microvascular endothelial function, and inhibits the viral cycle by increasing endosomal pH (5). In this study, we present a case of pediatric T1D that manifested with a hyperglycemic status requiring further management to maintain the glycemic threshold.
2. Case Presentation
A 9-year-old Afghan girl, a known case of type 1 diabetes mellitus (T1DM), is the focus of this study. Initially, she was admitted to our center, Amir-Al Momenin Hospital in Semnan, Iran, in January 2020 with a diagnosis of diabetic ketoacidosis (DKA) following uncontrolled T1DM. She was managed initially and discharged with a prescription for subcutaneous insulin. Unfortunately, the patient did not adhere to the follow-up visits and demonstrated poor compliance with glucose monitoring.
In January 2021, she was readmitted presenting with severe hyperglycemia (blood sugar (BS) > 750 mg/dL). Upon admission to the emergency department, she underwent primary assessments and fluid resuscitation. Her initial vital signs were as follows: Temperature of 36.8°C, pulse rate of 100 beats per minute, respiratory rate of 18 breaths per minute, blood pressure of 110/70 mmHg, and an oxygen saturation of 97% on room air. The pulmonary and cardiovascular examinations were normal, with no signs of abdominal distention, tenderness, or guarding. The patient was alert and oriented, and neurological assessments were normal. Notably, there were no clinical or laboratory signs of DKA, and further examinations revealed a stable condition. Table 1 represents laboratory investigations of the patient at admission.
| Parameters | At Admission | Normal Range |
|---|---|---|
| Arterial blood gases (ABG) | ||
| HCO3 (mmol/L) | 25.3 | 22 - 24 |
| pH | 7.414 | 7.25 - 7.35 |
| PaCO2 (mmHg) | 39.6 | 35 - 45 |
| Biochemistry | ||
| HbA1C (%) | 12 | ≤ 5.5 |
| BS (mg/dL) | 775 | 65 - 100 |
| BUN (mg%) | 14 | 6 - 21 |
| Cr (mg/dL) | 0.62 | 0.5 - 1.5 |
| AST (U/L) | 40 | < 31 |
| ALT (IU/L) | 68 | < 31 |
| ALP (IU/L) | 660 | 180 - 1200 |
| Na (mEq/L) | 131 | 135 - 145 |
| K (mEq/L) | 4.4 | 3.5 - 5.5 |
| Calcium (mg/dL) | 9.1 | 8.5 - 10.5 |
| Hematology | ||
| WBC (/µL) | 1.9 × 103 | 3.5 - 10 × 103 |
| Hb (g/dL) | 12.2 | 12 - 18 |
| Lymph (%) | 52 | - |
| PMNs (%) | 49 | - |
| Plt (/µL) | 186 × 103 | 150 - 450 × 103 |
| ESR (mm/h) | 17 | ≤ 20.0 |
| PTT (s) | 30 | 26 - 38 |
| INR | 1 | 0.8 - 1.2 |
Abbreviations: HCO3, bicarbonate; PaCO2, partial pressure of carbon dioxide; HbA1C, hemoglobin A1C; BS, blood glucose; BUN, blood urea nitrogen; Cr, creatinine; AST, aspartate Transaminase; ALT, alanine Transaminase; ALP, alkaline Phosphatase; Na, sodium; K, potassium; WBC, white blood cell; Hb, hemoglobin; Lymph, lymphocytes; PMNs, polymorphonuclear cells; Plt, platelet; ESR, erythrocyte sedimentation rate; PTT, partial thromboplastin time; INR, international normalized ratio.
Despite the administration of subcutaneous insulin, the patient manifested resistance to the treatment, prompting her transfer to the pediatric intensive care unit (PICU). She underwent intravenous insulin infusion therapy (0.1 unit/kg per hour, insulin regular, Exir, Iran). Concurrently, lymphopenia was noted, and a diagnosis of COVID-19 was confirmed by real-time polymerase chain reaction (RT-PCR). A chest computed tomography (CT) scan indicated minor viral engagement.
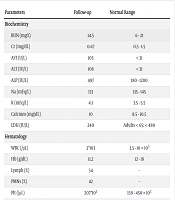
Unexpectedly, the patient did not respond to the intravenous insulin infusion, continuing to exhibit a resistant hyperglycemic status. The insulin dosage was increased to 0.15 unit/kg per hour, and oral metformin (500 mg twice daily, Chemi Darou, Iran) was introduced into the treatment regimen. Remarkably, the combination of metformin and insulin therapy led to a resolution of the hyperglycemic status within six days. Table 2 shows laboratory investigations.
Upon stabilization, the patient was transferred to the pediatric ward, and insulin administration was switched back to the subcutaneous route. She responded well to the adjusted blood glucose management (dosage 1.2 U/kg/day) and was discharged with instructions for ongoing subcutaneous insulin therapy. The parents were advised on potential emergency conditions and urged to attend the pediatric endocrinology clinic for evaluation and support in self-monitoring of blood glucose.
| Parameters | Follow-up | Normal Range |
|---|---|---|
| Biochemistry | ||
| BUN (mg%) | 14.5 | 6 - 21 |
| Cr (mg/dL) | 0.67 | 0.5 - 1.5 |
| AST (U/L) | 105 | < 31 |
| ALT (IU/L) | 108 | < 31 |
| ALP (IU/L) | 497 | 180 - 1200 |
| Na (mEq/L) | 133 | 135 - 145 |
| K (mEq/L) | 4.1 | 3.5 - 5.5 |
| Calcium (mg/dL) | 10 | 8.5 - 10.5 |
| LDH (IU/L) | 240 | Adults < 65: < 480 |
| Hematology | ||
| WBC (/µL) | 3 × 103 | 3.5 - 10 × 103 |
| Hb (g/dL) | 11.2 | 12 - 18 |
| Lymph (%) | 54 | - |
| PMNs (%) | 42 | - |
| Plt (/µL) | 207 × 103 | 150 - 450 × 103 |
| ESR (mm/h) | 25 | ≤ 20.0 |
Abbreviations: BUN, blood urea nitrogen; Cr, creatinine; AST, aspartate transaminase; ALT, alanine transaminase; ALP, alkaline phosphatase; Na, sodium; K, potassium; LDH, lactate dehydrogenase; WBC, white blood cell; Hb, hemoglobin; Lymph, lymphocytes; PMNs, polymorphonuclear cells; Plt, platelet; ESR, erythrocyte sedimentation rate.
3. Discussion
High prevalence of diabetes has been observed among severe cases and fatalities due to SARS-CoV-2 viral infection. Various mechanisms have been hypothesized to investigate this phenomenon, including potential damage to pancreatic β-cells, a higher rate of obesity among diabetic patients, formation of systemic inflammation and cytokine storms, presence of comorbidities such as hypertension, cardiovascular and renal diseases, hyperglycemia itself, and coagulopathy (6).
At the onset of the COVID-19 pandemic, it was observed that children with type 1 diabetes mellitus (T1DM) could present with diabetic ketoacidosis (DKA) as a complication of SARS-CoV-2 infection. Han and Heo demonstrated that the incidence of pediatric DKA was magnified during the COVID-19 pandemic in South Korea, with most cases presenting non-specific symptoms (7). Although the prevalence of T1DM did not change significantly during the outbreak, the incidence of DKA in pediatric T1DM increased (8). A global survey concluded that this alteration could be related to quarantine policies, limited accessibility to exercise and medical facilities, restrictions in routine follow-ups, delays in initial interventions, and a significant increase in the rate of DKA presentation (9).
Several questions remain unclear. However, the SARS-CoV-2 virus may directly disturb pancreatic β-cell activity through interaction with the angiotensin-converting enzyme 2 (ACE2) receptor. Moreover, an increased rate of insulin resistance in T1D patients, leading to chronic metabolic disorders that were non-existent prior to infection, is a broadly accepted concept (10). We observed a condition of insulin resistance without the presence of DKA in a T1DM patient. In Pediatric Intensive Care Unit (PICU) management, an increase in the dosage of intravenous insulin therapy combined with metformin improved the hyperglycemic status. The International Society for Pediatric and Adolescent Diabetes (ISPAD) also suggests a similar range of insulin doses (11). Additionally, in moderate hospitalized cases similar to our patient, the administration of metformin has been suggested as well (12).
Metformin is recognized for its anti-inflammatory, cardio-protective, and antiviral effects (13). An expanding body of research demonstrates that metformin plays a significant role in reducing disease severity and inflammatory responses in SARS-CoV-2 patients. In vivo studies have shown that metformin disrupts the interaction between host and viral proteins necessary for viral replication, virion assembly, and pathogenesis (14). It also exerts immunomodulatory and anti-inflammatory effects and reduces insulin resistance (15, 16). In SARS-CoV-2 infection, virus binding to the ACE2 receptor and subsequent decrease in ACE2 availability leads to hyper-activation of the angiotensin II/AT1R axis and activation of NF-κB, which mediates inflammatory processes and production of pro-inflammatory cytokines (TNFα, IL-6, IL-1, and IL-1β) (17). Metformin prevents IL-6 release by increasing the AMP/ATP ratio, subsequently inhibiting mTOR and further suppressing ROS-induced oxidative stress (18).
A limitation of the present study was the lack of measurement of inflammatory markers. In conclusion, the insulin resistance condition, well established in SARS-CoV-2 infection, could be effectively managed through the administration of metformin and intravenous insulin (regular) combination therapy. Further studies are required to clarify this controversial condition.