1. Background
Cereals and legume crops are primary components of many diets in developing countries, serving as valuable sources of essential nutrients such as proteins and minerals. Whole grain products are recommended for their potential therapeutic benefits, including combating cardiovascular diseases, diabetes, and cancer (1). These products contain phytic acid, the main storage form of phosphorus in cereals, comprising 70 - 80% of the total phosphorus and 3 - 5% of the seeds' dry weight (2). Commonly viewed as an anti-nutrient in monogastric animals' diets, phytic acid contributes significantly to phosphorus pollution in animal manure. It binds with important mineral ions, proteins, and amino acids, reducing the availability of essential nutrients and hindering starch assimilation (3). To address phytate-related issues, enzymatic degradation of phytate is a preferred alternative to costly physical and chemical methods that diminish nutritional value (4).
Phytases, also known as myo-inositol hexakisphosphate phosphohydrolase (EC 3.1.3.8), belong to the histidine acid phosphatase (HAP) family. They catalyze the hydrolysis of phytic acid into myo-inositol and phosphoric acid, converting penta- to mono-phosphates. This process enhances the bioavailability of essential minerals and mitigates environmental pollution from excessive use of inorganic phosphorus supplements (5). The enzymes find widespread application in the food industry, livestock, and other sectors due to their crucial role in degrading phytate (6).
Recent years have seen a growing interest in enzymatic hydrolysis of phytate, driven by rising demand for functional foods and sustainable agricultural methods. In the USA, phytase sales reached approximately $150 million annually, constituting one-third of the total enzyme market (7). Optimizing phytase production is essential due to its growing demand. Various microorganisms have been evaluated for their phytase production capabilities using a phytase screening medium (PSM) supplemented with substrates like calcium phytate and sodium phytate (8, 9). The choice of microorganism depends on the substrate, environmental conditions, and desired end product. Phytase production has been thoroughly researched in plants, animals, and yeast. However, the existence and attributes of phytase-producing fungi in processed cheese, a widely consumed dairy product, have not been extensively investigated.
Processed cheese, known for its distinctive flavor and texture, may harbor fungi capable of producing phytase (10). Exploring these fungi's enzymatic capabilities could reveal efficient new methods for producing phytase in the food industry and expand its use in animal feed, human nutrition, and biotechnology. Fungi are considered optimal for commercial phytase production due to their ease of cultivation and high enzyme yields, surpassing other sources (11). Currently, only a few microorganisms are involved in the commercial production of phytases, highlighting the importance of exploring new fungal strains as phytase producers. Fungi are recognized for their capacity to produce extracellular enzymes, as opposed to the intracellular enzymes produced by yeast and bacteria, leading to favorable operational costs, particularly on an industrial scale. Expanding the sample size and diversity of fungal isolates, as well as studying the enzyme's efficacy in different substrates beyond ultra-refined white cheeses, might reveal potential applications in various food formulations.
This study's hypothesis suggests that ultra-refined white cheeses contain a variety of fungal species capable of producing phytase enzymes, which possess distinctive enzymatic properties suitable for potential industrial use. Our objectives include expanding the understanding of phytase enzymes, discovering new sources of enzymes, and facilitating the industrial application of these enzymes across different sectors.
2. Objectives
This study aims to enhance the comprehension and utilization of phytase enzymes in the industrial sector by isolating, screening, and characterizing phytase-producing fungi from processed cheese, selecting the most efficient producer, evaluating their enzyme activity, and determining optimal conditions for high enzymatic production.
3. Methods
3.1. Samples
Cheese samples were collected from a local store in Shiraz, Iran, and transported to the microbiology lab in sterilized polyethylene bags where they were stored at 4°C. The salt of phytic acid was sourced from Sigma-Aldrich (USA). Culture media such as potato dextrose agar (PDA), water agar (WA), PSM, and Luria Bertani broth (LB) were provided by the Institute of Standards and Industrial Research of Iran. All other chemical agents were domestically produced and of analytical grade.
3.2. Fungi Isolated from Processed Cheeses
The isolation technique described by Samson et al. (2010) was used to isolate fungi from the cheese samples. Out of the 110 collected samples, 20 were stored with the door open and 90 with the door closed to facilitate fungal isolation. The samples were directly placed onto PDA media supplemented with chloramphenicol in triplicate, with five pieces per dish, and incubated at 25°C for 5 to 7 days. Each resulting fungal colony was then transferred to fresh PDA and further incubated under the same conditions for additional experiments. The purified samples were sequenced, and the data were submitted to the World Gene Bank (www.ncbi.nlm.nih.gov) for genus and species classification.
3.3. Fungi Purification
The purification process began with the gentle extraction of the isolated fungus using a sterile needle, which was then placed on a WA medium. These Petri dishes containing the fungi were positioned in a controlled environment such as an incubator, set at a temperature of 25°C. After approximately 2 to 3 days, a segment of the fungal thread was carefully removed using a sterile needle and transferred to a new medium, PDA. This was performed near an open flame to facilitate regrowth (11).
3.4. Fungi Identification
To ascertain the identity of the purified fungi, both morphological and molecular techniques are utilized:
- Morphological identification: A slide is prepared with a piece of mushroom mycelium obtained using a sterile needle, placed in a water droplet, and covered with a microscope slide. Under the microscope, the spore characteristics, coloration of reproductive organs, and an identification key for incomplete fungi are examined to determine the fungus's genus (5, 12).
- Molecular identification: For DNA extraction, three blocks of isolated and purified fungi are transferred to 250 mL flasks containing 50 mL of sterilized potato extract. These flasks are incubated at 25°C for 10 days. The contents are then transferred to sterile Petri dishes and rinsed with distilled water. The mycelium, separated from the agar using sterile needles, is placed into 1.5 mL microtubes, frozen in liquid nitrogen, and subjected to a 24-hour freeze-drying process (13). The dried mycelia are then ground, and DNA is extracted for analysis. Polymerase chain reaction (PCR) is conducted using primers ITS1 (TCCGTAGGTGAACCTGCGG) and ITS4 (TCCTCCGCTTATTGATATGC), with PCR products undergoing electrophoresis in a 1% agarose gel. The samples are sequenced post-purification, revealing the genetic information of the fungi (13).
3.5. Isolation of Phytase-Producing Fungi
To isolate phytase-producing fungi, samples were cultured on PSM media and incubated at 28 - 30°C for 96 hours (14). Phytase screening medium is designed to evaluate enzyme activity. The presence of a bright halo around the fungi indicates their ability to produce phytase and utilize sodium phytate. Phytase screening medium ingredients include Na-phytate (0.5%), glucose (1.0%), (NH4)SO4 (0.03%), MgSO4 (0.05%), CaCl2 (0.01%), MnSO4 (0.001%), FeSO4 (0.001%), and agar (2.0%), with a pH of 7.0.
3.6. Phytase Enzyme Assay
The phytase activity of each isolate is quantified using sodium phytate as a substrate and measuring the release of free phosphate. The assay procedure includes the following steps: 10 microliters of enzyme solution are added to 350 microliters of 100 mM sodium acetate buffer (pH 4.5) containing 875 nmol of sodium phytate. After incubating at 37°C for 30 minutes, free phosphorus is determined using the molybdate method (15) with modifications. The assay mixture comprises 1.5 mL of fresh acetone solution, 5 NH2SO4, 10 mM ammonium molybdate (v/v ratio 1:1:2), and 100 microliters of 1% citric acid. The sample is centrifuged at 15,000 g for 2 minutes, and the absorbance is read at 700 nm using a spectrophotometer (Shimadzu UV-VIS 1601, Japan) (16). A unit of phytase activity is defined as the amount capable of liberating 1 nanomole of inorganic phosphate per ml per second, using KH2PO4 as a standard.
3.7. Temperature and pH Optima of Phytase Enzyme
The microorganisms of interest were cultured in LB liquid medium and incubated for four days. After incubation, 1000 microliters of the medium were centrifuged at 6000 rpm for 3 - 4 minutes. Following centrifugation, a filtration step was conducted, and the resulting liquid was used for experiments. The enzyme activity was then assessed across a range of pH values (2.5 - 9) and temperatures (30 - 90°C) to determine the optimal conditions for phytase activity (13).
3.8. Statistical Analyses
The collected data were analyzed using one-way analysis of variance (ANOVA) and post hoc multiple comparison tests (LSD) utilizing SPSS version 19.0 (USA). The experiment was conducted three times on two separate occasions (n = 6), and the average value of the results was calculated.
4. Results
This study was driven by the hypothesis that fungal isolates from ultra-refined white cheeses might possess the capability to produce phytase enzymes with distinctive enzymatic traits. Our research methodology focused on evaluating enzyme activity levels, defining the optimal environmental conditions for enzyme production, and characterizing the enzymatic profiles of specific isolates. Through a systematic analysis of these parameters, our goal was to clarify the enzymatic potential of the identified fungal species and to assess their suitability for industrial applications. Microorganisms, as a diverse group, serve as a reliable source of numerous beneficial products, including enzymes. This endeavor contributes significantly to advancing knowledge in the field of phytase enzyme production and utilization.
The initial results from isolating fungi from cheese indicated that eight types of molds were isolated, as shown in Table 1. All 20 cheese samples stored with the door open were contaminated with fungi. Penicillium was the most prevalent fungus, accounting for 45% of the contamination. This was followed by Aspergillus, which occurred in 30% of the samples, while Paecilomyces and Trichoderma accounted for 15% and 10% of the contamination, respectively. Prior research by Kandasamy et al. (17) highlighted that Penicillium and Aspergillus molds are frequently linked to significant levels of contamination in cheese. Among the 90 packages of cheese samples, 19 (21.1%) were found to be contaminated with fungi. Byssochlamys spectabilis was the most common, affecting 52.6% of the contaminated samples, followed by Aspergillus oryzae, Cladosporium, Cladosporioides, and Aspergillus niger with occurrences of 21%, 15.78%, and 10.52%, respectively.
| Microorganism | Percentage |
|---|---|
| Door open | |
| Penicillium | 45 |
| Trichoderma | 10 |
| Paecilomyces | 15 |
| Aspergillus | 30 |
| Door close | |
| Byssochlamys | 52.6 |
| Cladosporioides | 10.52 |
| Cladosporium | 15.78 |
| Aspergillus oryza | 21 |
4.1. Monitoring of Phytate-Degrading Activity in Solid Medium
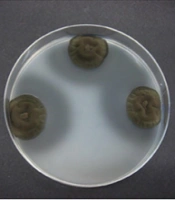
In the initial screening program for the isolation of phytase-producing fungi, 110 fungal isolates were collected from processed cheese samples. All obtained fungal isolates were introduced to PSM plates, which contained phytic acid as the primary phosphorus source, to observe their growth. After the incubation period, the majority of the fungal isolates thrived on the PSM agar plates, with only a few exceptions that did not grow. Notably, only a small number of fungal strains demonstrated the formation of a clear zone surrounding their colonies, an indication of the de-phosphorylation of sodium phytate. Figure 1 illustrates the zone of inhibition around colonies of some isolates. From the initial pool of 110 isolates, 28 exhibited a remarkable ability to produce phytase, as evidenced by the presence of a halo zone around their colonies. These isolates were subsequently selected for further evaluation of their potential to generate extracellular phytase in a liquid culture setting.
4.2. Monitoring of Phytate-Degrading Activity in Liquid Medium
Phytase activity was assessed by measuring the quantity of inorganic phosphate released and its subsequent reaction with a color reagent. While using a solid medium to measure extracellular phytate-degrading activity can potentially lead to false-positive outcomes, it is crucial to validate these results in a liquid medium to ensure accuracy. Initial findings indicated that 28 out of the 110 fungal isolates previously selected were capable of hydrolyzing phytate in liquid PSM. Consequently, only those isolates displaying significant phosphorus-solubilizing capability in liquid culture were included in this study. Among these, 6 fungal strains that exhibited the most promising phytase production were chosen for further examination. The phytase activity of these 6 strains was quantified, highlighting one strain with the highest activity.
These 6 isolates showed phytase activity in both PSM and LB media, as documented in Table 2. The identified fungal species included A. niger, A. oryzae, P. commune, P. chrysogenum, P. variotii, and C. cladosporioides. Previous studies by Howson and Davis (9) also observed that strains from various genera such as Aspergillus, Rhizopus, Mucor, and Geotrichum produced phytases in both PSM and potato dextrose broth.
Table 2 clearly shows that all six isolates had the capacity to utilize sodium phytate and generate phytase. Notably, all isolates recorded their highest enzymatic activity when using LB media compared to PSM media. In the current study, phytase activities of cell-free supernatant from isolates grown in LB media ranged from 72.3 to 216.7 U/mL, which aligns with previous reports. For example, Monteiro et al. (18) documented the production of phytase by Aspergillus niger UFV-1 with an enzyme activity of 138.6 U/mL. Rani and Ghosh (19) also reported the presence of the phytase enzyme in A. oryzae. Remarkably, this study is the first to document phytase production by P. variotii. The highest phytase activity was observed in P. commune and P. variotii in PSM and LB medium, respectively. The isolate P. variotii, displaying the maximum production in both LB and PSM media as shown in Table 2, was selected for further investigation.
| Fungi Species | Enzyme Activity in LB Media (U/mL) | Enzyme Activity in PSM Media (U/mL) |
|---|---|---|
| Aspergillus niger | 72.3 | 68 |
| Aspergillus oryza | 127.02 | 106.31 |
| Penicillium commune | 187.72 | 133.76 |
| Penicilliumchrysogenum | 98.89 | 82.6 |
| Paecilomycesvariotii | 216.7 | 124.1 |
| Cladosporiumcladosporioides | 78.7 | 28.3 |
4.3. pH and Temperature Optimization of the Extracellular Phytate-Degrading Enzyme
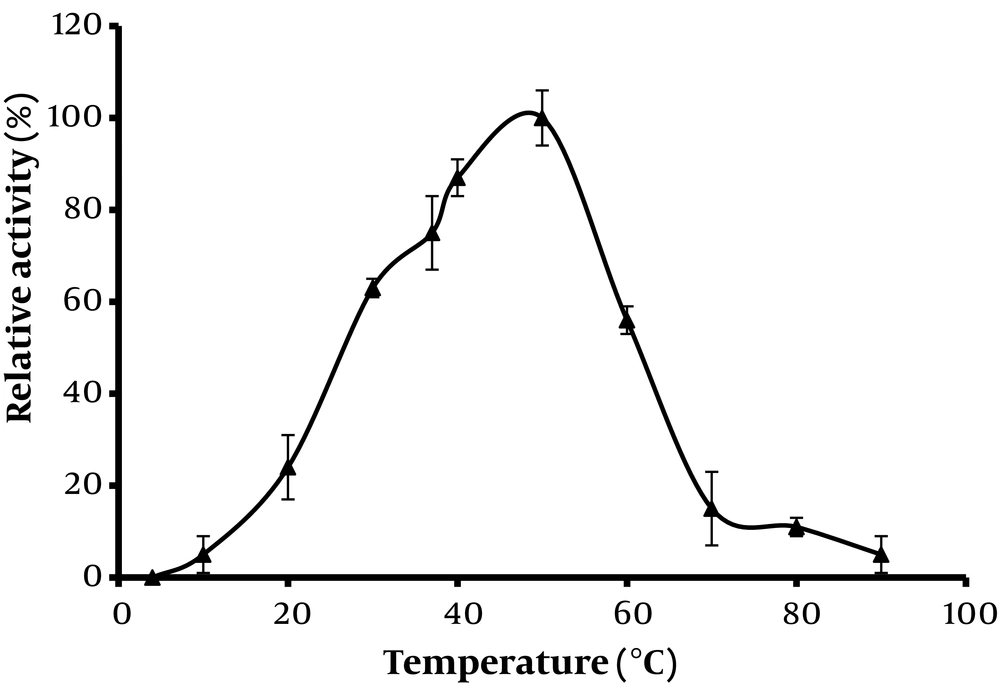
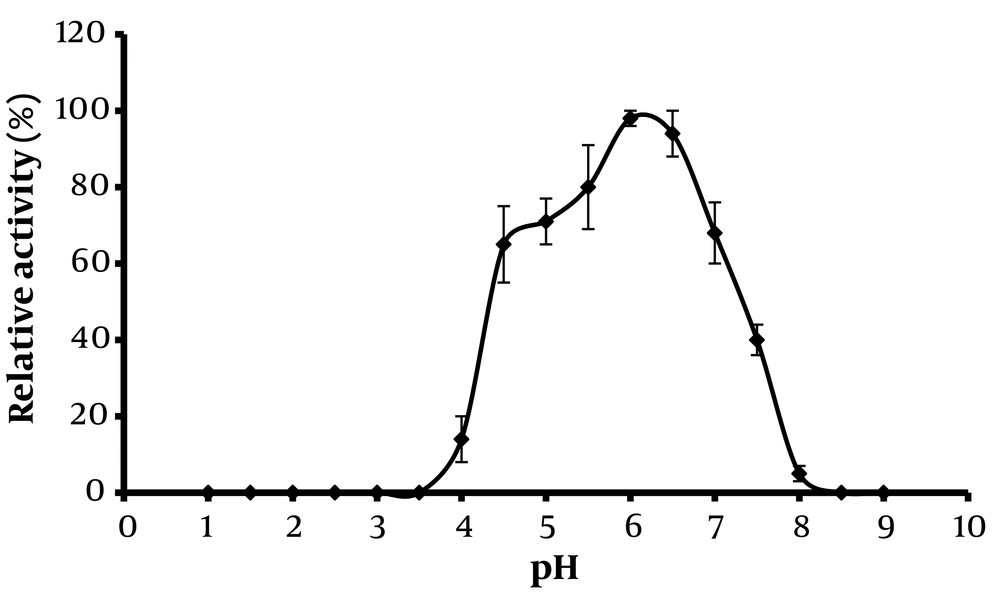
Phytase enzymes sourced from various organisms often display a wide range of characteristics, which significantly influence their industrial applicability. Figures 2 and 3 demonstrate the optimum activity of extracellular phytase from Paecilomyces variotii across different temperatures and pH levels, respectively.
The thermal dependence of phytase activity, crucial for its application, is highlighted in Figure 2. The findings indicate that phytase activity increased when temperatures were raised from 30°C to 50°C. However, further increases in temperature led to a decline in activity, likely due to heat-induced denaturation of the enzyme. The peak activity of phytase was observed at 60°C, suggesting this as the optimal temperature. This temperature is consistent with findings for Aspergillus flavus (20), Rhizopus oryzae (19), and Fusarium verticillioides (21), but lower than those reported for Rhizopus oligosporus (22) and Aspergillus niger (12).
Regarding pH stability, phytase activity increased as pH values rose from 2 to 6, as depicted in Figure 3. Beyond pH 6, there was a sharp decline in activity, and at pH values of 8.2 and above, the enzyme's activity was completely lost, likely due to alkaline-induced denaturation. The optimal pH for phytase activity was determined to be 6. Under extremely alkaline or acidic conditions, the enzyme lost activity, likely due to structural alterations in the phytase proteins. The enzyme showed robust stability across a wide pH range, maintaining constant activity throughout the pH spectrum. This optimal pH aligns with previous studies (20, 22) and is relevant for various sections of the digestive tract—salivary glands (pH 5), stomach (pH 2 - 4), and small intestine (pH 4 - 6) (23). The results clearly demonstrate that the enzyme maintains a favorable level of activity within the digestive tract, underscoring its potential utility in dietary applications.
5. Discussion
Enzyme heat resistance is a critical consideration in the industrial selection of enzymes. Phytases, derived from various microorganisms including fungi, demonstrate a broad spectrum of characteristics that have been extensively studied. Extracting microbial enzymes from wild species is a viable method for discovering innovative enzymes. Typically, the optimal temperature for most known phytases ranges from 37°C to 70°C (24).
The high temperature optimum poses challenges for phytases, potentially inhibiting their activity in the stomach temperatures of poultry (37 - 40°C) and impacting performance in fish. Despite these challenging conditions, the heat resistance of phytase from P. variotii is notable, maintaining 90% of its activity even after exposure to 70°C for 10 minutes. Phytases like those from P. variotii, which exhibit high thermal stability, offer significant advantages in commercial feeds that undergo pelleting at high temperatures (60 - 80°C).
Concerning pH, most known phytases function optimally within a pH range of 4.5 to 6.0 (24). Histidine acid phosphatase-type phytases operate effectively in the acidic upper regions of an animal's digestive system, while ß-propeller phytases are active in the lower sections, such as the small intestine, where neutral to alkaline conditions are prevalent. The stomach's ability to sustain low pH levels is crucial, as HAP phytases show stability in acidic conditions, and β-propeller phytases demonstrate exceptional stability at higher pH levels. Phytases are categorized as acid phytases, with an optimal pH range of 3.5 - 6.0, and alkaline phytases, with an optimal pH of 7.0 - 8.0. Alkaline phytases have been isolated from the P. variotii species.
The catalytic activity of phytases increases with temperature up to an optimum point, beyond which further increases result in heat-induced denaturation and a decline in activity. The optimal temperature for phytate degradation varies depending on the enzyme source, ranging from 35°C to 90°C. The phytase from P. variotii shows residual activities of 75%, 62%, and 41% when incubated at 80°C for 10, 20, and 30 minutes, respectively. pH stability studies indicate a dramatic decrease in activity at pH 6.0, with only 1% of the original activity remaining after 30 minutes. The highest stability is observed at pH 6. Overall, phytases from P. variotii are highly thermostable enzymes with potential applications as supplements in food and feed.
5.1. Conclusions
In this study, phytase enzyme-producing fungal species were successfully identified among the contaminant isolates from ultra-refined white cheeses. The research revealed that 60% of the isolated fungal samples had the capacity to produce the phytase enzyme. Notably, this included new insights into the phytase production capabilities of P. variotii. The enzyme activities of P. commune and P. variotii showed considerable potential, with the latter being chosen for further research due to its superior properties. The optimal temperature and pH for the phytase enzyme produced by P. variotii were identified as 50°C and pH 6, respectively. These findings underscore the distinctive attributes of this enzyme and emphasize its potential for industrial applications. The study suggests that the phytase enzyme from P. variotii could be a valuable resource for various industries, facilitating its path toward industrialization and broadening the scope of industrial phytase production. While many studies focus on the production and characterization of phytase from certain microorganisms, further investigation into enzyme characteristics such as regulation, catalytic capacity, specificity, and optimization of production is necessary to decrease costs and enhance the practical application of this enzyme on an industrial scale.