1. Background
Aflatoxins (AFs) are a group of fungal toxins (mycotoxins) produced by Aspergillus strains that have been associated with various harmful health effects in humans and animals (1, 2). Certain strains of Aspergillus flavus, Aspergillus parasiticus, and Aspergillus nomius are capable of producing AFs during growth on various food and feed sources throughout the entire food processing chain, from farm to table. Aflatoxin B1 (AFB1) is the most toxic form of AF and is considered one of the strongest natural mutagens and carcinogens known to humanity (3). Through a direct metabolic pathway, AFB1 and AFB2 are converted into other types of AFs, specifically aflatoxin M1 (AFM1) and aflatoxin M2 (AFM2), in the bodies of livestock. AFM1 and AFM2 are then metabolized and excreted in milk when animals consume AF-contaminated feed (4-6). Consequently, milk and dairy products, as well as breast milk, can contain AFM1, posing a hazard and a means of transmission to consumers. Given the widespread consumption of milk and dairy products as nutritious foods, awareness of the possibility of AFM1 contamination and the risk of exposure, particularly in children, has increased (4, 7).
The International Agency for Research on Cancer (IARC) classifies AFM1 as a Group 2B chemical, indicating that it is possibly carcinogenic to humans. While AFM1 is approximately 10 times less carcinogenic than AFB1, its exact mechanisms of toxicity in humans are not yet fully understood (8, 9). However, it has been proven that AFM1 may induce liver cancer through a mechanism similar to that of AFB1. Unlike AFB1, AFM1 appears to cause cytotoxicity in human cells independently of metabolic activity (10). Some reported health-related effects of AFs include liver cirrhosis, tumor development, immunosuppression, mutation, fetal malformation, and cancer (9).
The Food and Drug Administration (FDA) has established specific guidelines to regulate acceptable levels of aflatoxins in food and feed. These guidelines set thresholds that require the rejection of contaminated products containing AF levels potentially higher than the maximum tolerated level. The European Committee and Codex Alimentarius have set a limit of 50 ng Kg-1 for AFM1 in milk (11). Given the aforementioned hazards associated with the presence of AFM1 in milk, it is urgent to monitor the AFM1 content and protect the population from exposure (12). Several studies have outlined various methods to reduce aflatoxin contamination and assess its risks (3). These approaches include chemical or physical AF degradation methods such as thermal processing, irradiation, and fumigation. Additionally, the use of adsorbent or binding agents can modify or remove mycotoxins and their metabolites from the food matrix (13).
Currently, research is ongoing to investigate biological strategies for AF decontamination using recognized safe microorganisms (14, 15). Multiple studies have demonstrated the capacity of certain bacteria and yeast to effectively eliminate aflatoxins in aqueous media through both in vitro and in vivo simulators (16-19). In this context, innovations aimed at reducing the health risks of AF in food include using specific probiotic strains as efficient binders for AFs, helping to eliminate them from the body. Numerous studies have reported that certain lactic acid bacteria (LAB) and yeast strains, recognized as probiotic microorganisms, have the capacity to adsorb/bind AF molecules via cell wall components, forming a stable AF-microorganism complex (20). This process sequesters AF, degrading it into less toxic components (14). Several studies have successfully applied probiotic strains to decontaminate AFM1 from milk and dairy products (18, 21, 22).
Khiki cheese is a type of traditional cheese made by local residents of Iran using ruminant milk (mainly sheep and goat) without starter culture. The cheese curd is formed by adding a cheese starter, which originates from the abomasum and acts as rennet. The ripening/aging process occurs in bags made from livestock skin over a period of one month. This cheese is predominant due to its unique organoleptic properties. As traditional and cottage dairy products like cheese contain new sources of probiotic strains, isolating and evaluating their biological efficiency can contribute to safer food production. Furthermore, adding isolated and identified probiotic microorganisms from dairy products to milk or other dairy types could develop bio-enhanced food products and detoxify potential AFM1 contamination (23-25).
2. Objectives
The aim of this research was to assess the potential of LAB strains isolated from a type of cottage cheese called Khiki for binding AFM1 in PBS and reducing its content in artificially contaminated milk.
3. Methods
3.1. Materials
The standard AFM1, with a purity level of 98%, was purchased from Sigma (St. Louis, MO). To create a standard suspension, a specific amount of AFM1 was dissolved in a 1: 1 ratio of ethanol to water (v/v) and used for spiking the phosphate-buffered saline (PBS) and milk. Ultra-high-temperature (UHT) cow's milk was purchased from a local market in Semnan province, Iran (35.5769°N, 53.3953°E), and a thermal process (100°C) was performed before analysis. All other reagents used were of analytical grade.
3.2. Bacteria Strains
The LAB strains isolated and identified from an Iranian cottage cheese (Khiki) in a previous study by the author were used in this research. In brief, all gram-positive and catalase-negative bacteria isolated from fourteen Khiki cheese samples (collected from the local area of Semnan province) were analyzed, and LAB strains were genetically identified. According to 16S rRNA sequencing, the dominant Lactobacillus species identified were L. plantarum, L. paracasei, and L. casei (26). For the present study, each isolated LAB strain was cultivated in De-Man-Rogosa-Sharpe (MRS) broth and incubated for 24 hours at 37°C under anaerobic conditions in a shaker incubator. The bacterial pellets were obtained by centrifuging the culture tubes at 4000 rpm for 15 minutes, followed by washing twice with PBS. Each pellet of LAB strains was then suspended in PBS, and its optical density was determined at 600 nm. The concentration of the bacterial suspension was adjusted to 1 × 109 CFU/mL-1 in PBS.
3.3. Aflatoxin Binding Assay in PBS
The bacterial strains (1 × 109 CFU/mL-1) were inoculated in separate microtubes, each containing 1 ml of PBS spiked with AFM1 (50 ng/mL-1), and incubated at 37°C for 12 hours. A microtube containing only PBS and AFM1 was used as a negative control. After the incubation period, all tubes were centrifuged at 3000 g for 15 minutes. The resulting supernatants were collected and transferred to clean tubes, then stored at 4°C until AFM1 analysis (24).
3.4. Aflatoxin Binding Assay in Milk
Before the experiment, it was confirmed that the sterile milk samples did not contain any AFM1 contamination. Following this, 200 mL of milk were inoculated with a suspension of LAB cells at a concentration of 109 CFU/mL-1, along with 50 ng/mL-1 of spiked AFM1. The samples were then incubated at 4°C for 1 hour. After incubation, the samples were centrifuged at 3000 g for 15 minutes, and the supernatants were collected for AFM1 analysis. To investigate the binding of AFM1 by bacterial strains over time, the strain with the highest potential for AFM1 binding in the initial assay was selected for a contact time experiment. The milk samples containing bacteria and AFM1 were incubated for 1, 12, 24, and 48 hours, and after each period, the samples were centrifuged, and the AFM1 levels in the supernatant were analyzed (14).
3.5. Aflatoxin M1 Analysis
The AFM1 content in the samples was quantified using high-performance liquid chromatography (Waters e2695, Blue series, USA) equipped with a C18 column (5 µm, 4.6 × 150 mm, GL Sciences, Japan) and a fluorescence detector (Waters 2475, USA). The mobile phase consisted of water and methanol in a 60: 40 ratio, with a flow rate of 2 mL/min. The excitation and emission detection wavelengths were adjusted at 365 nm and 435 nm. The limit of detection (LOD) and limit of quantification (LOQ) were 0.3 and 1 µg mL-1 respectively.
3.6. Statistical Analysis
The data collected from each experiment were measured at least three times and are presented as the mean ± standard deviation (S D). To determine differences between the samples, we conducted a one-way analysis of variance (ANOVA) followed by Duncan's test using SAS software. A significance level of P < 0.05 was considered statistically significant.
4. Results
4.1. Aflatoxin M1 Binding by Probiotic Bacteria in PBS and Milk
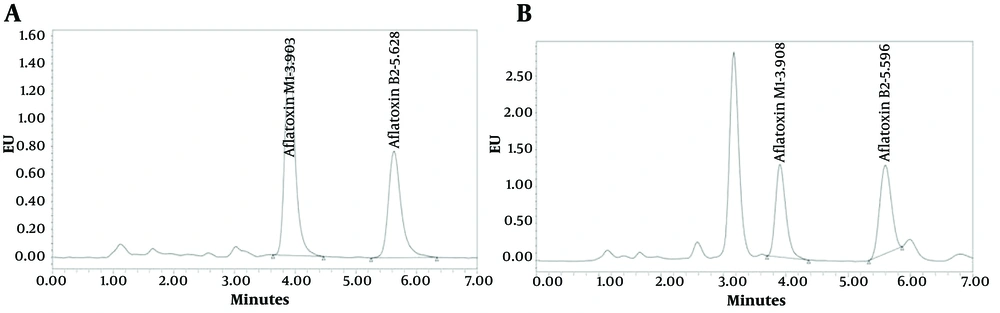
The AFM1 reduction by the probiotic bacteria isolated from an Iranian traditional cheese (Khiki) is shown in Table 1. All tested LAB strains were able to adsorb or bind AFM1 in PBS, ranging from 53.5% to 71.35% (P < 0.001). L. paracasei showed the highest reduction percentage (71.35%), followed by L. plantarum and L. casei. All assessed strains were also able to reduce AFM1 in milk, which served as a real food medium. As shown in Table 1 L. paracasei was able to bind the highest amount of AFM1 in milk (68.53%). Based on the obtained results, the rank order of AFM1 reduction by the studied probiotic bacteria was consistent in both PBS and milk. The peak area in the chromatogram obtained by HPLC analysis of the samples (Figure 1) illustrated the reduction of AFM1 concentration, with the peak area of AFM1 in a sample treated with L. paracasei being notably smaller than that of the PBS sample containing no LAB.
4.2. Aflatoxin M1 Binding by Probiotic Bacteria During Contact Time
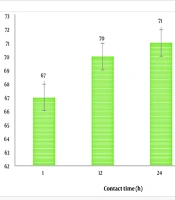
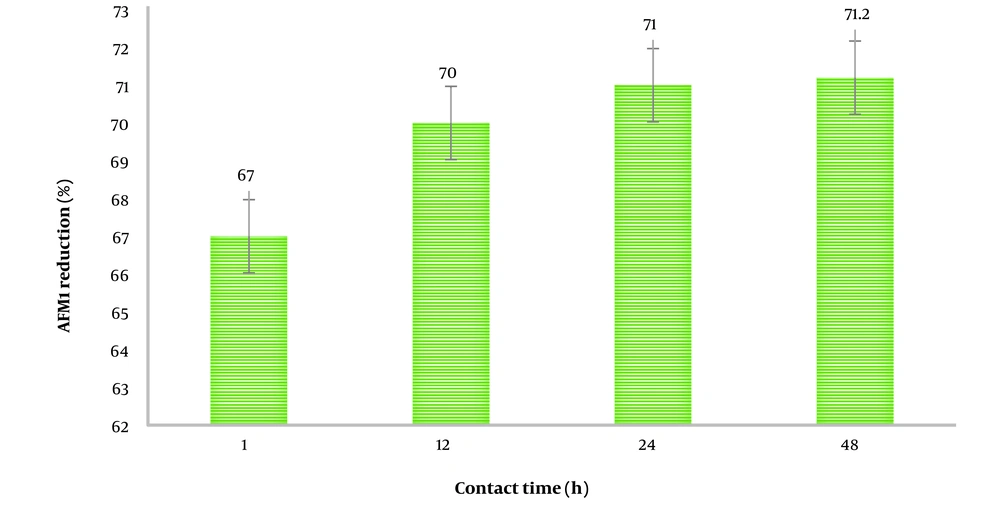
Since L. paracasei showed the highest AFM1 adsorption potential, it was used to evaluate the pattern of AFM1 reduction over different contact times. The AFM1 reduction rates in milk samples containing L. paracasei incubated for varying durations are shown in Figure 2. The greatest reduction (67 to 71.2%) occurred during the 1 to 48-hour contact period between L. paracasei and milk. The first 12 hours of contact time were particularly effective in the overall adsorption of AFM1 by the studied strains.
5. Discussion
In the present study, the LAB strains used were isolated from a traditional cheese named Khiki, produced in Semnan, Iran, and identified by 16S rRNA in the authors' previous work. Based on biochemical and physiological tests, the dominant isolated Lactobacillus species included Lactobacillus plantarum, Lactobacillus paracasei, and Lactobacillus casei. Since these microorganisms are recommended for use as starter cultures in cheese making, their AFM1 reduction potential could enhance product safety and help mitigate the harmful effects of AFM1 on humans. Given that exposure to AFM1 occurs through the consumption of contaminated milk and dairy products, applying new bio-based technology to reduce contaminant content would be beneficial (5, 27).
As observed in this study, the efficiency of different LAB strains varied, even within the same family. Therefore, selecting the most effective strain for AFM1 binding would yield greater health-promoting results (28). The AFM1 binding capacity of microorganisms has been attributed to cell wall components, with key components being polysaccharides and peptidoglycans that contain binding sites (22). Some researchers have shown that differences in AF adsorption ability depend heavily on the quantity of binding sites in the target microorganism (6, 12, 21).
In the study by Bueno et al., it was indicated that, during the binding event, a reversible complex forms between AFB1 and the surface of LAB strains or Saccharomyces cerevisiae, with no chemical interaction occurring (29). Elsanhoty et al. reported that the capacity of selected probiotic strains, including LAB and bifidobacteria, to reduce AFM1 in MRS broth and yogurt made from AFM1-spiked milk varied significantly. The highest binding was achieved by a starter culture that included S. thermophiles, L. bulgaricus, and L. plantarum (23).
In a study by Serrano-Niño et al., AFM1 detoxification by five probiotic strains varied by strain and ranged from 19.95 to 25.43% in PBS and 22.72 to 45.17% in a digestive model (30). Martínez et al. demonstrated that the probiotic strains Pediococcus pentosaceus and Kluveromyces marxianus were capable of reducing AFM1 by 19 - 61% in milk. They also revealed that AFM1 was degraded into metabolites that were less toxic than the aflatoxin molecule and thus harmless (14).
The study of biological reduction of AFM1 using probiotic microorganisms (Streptococcus thermophilus, Lactobacillus delbrueckii, Lactobacillus acidophilus, Bifidobacterium animalis, Lactobacillus casei, and Lactobacillus rhamnosus) in a traditional dairy product from Iran showed that Lactobacillus acidophilus was the most efficient binder of AFM1. Additionally, inoculating this strain during the fermentation process had a notable health-promoting effect (15). Supporting our results, Abbes et al. reported that lactic acid bacteria L. plantarum and L. rhamnosus, isolated from Tunisian artisanal butter, were able to reduce AFM1 in PBS and reconstituted milk by 15.3 - 95.1%, with L. rhamnosus showing a better potential for removal than L. plantarum (25).
Regarding the impact of contact time on the efficiency of strains to bind AFM1 in milk, it was found that the most adsorption (67%) occurred in the initial hours of contact. It appears that after the initial contact between the microorganisms and the AFM1-containing media, the majority of binding sites in the cell walls of the strains become saturated with AFM1 molecules. Consequently, no further physical adsorption occurs with extended contact. Therefore, there is no need to prolong the treatment with microorganisms for additional AFM1 detoxification. In other words, the binding that occurs during the initial contact period would be beneficial in food production, where microorganisms are used as additives. Our results on the contact time of strains with milk for AFM1 reduction are consistent with findings from other researchers in this field.
One of the strengths of traditional dairy products from various regions around the world is their microbiological diversity. Several isolated strains from dairies have been identified and their biochemical, physiological, and phenotypic properties determined. Additionally, the probiotic potential of these isolated strains has been analyzed. These probiotic microorganisms offer numerous health benefits, which have attracted researchers to study them. Cottage cheese from different local areas of Iran, produced from milk and non-industrial starters, contains unique strains that could serve as new sources of starter cultures for dairy products. As observed, these LAB strains exhibited a remarkable ability to reduce AFM1 in milk, making products containing these microorganisms appear safer for consumers. The industrial production and widespread marketing of probiotics could contribute to offering healthier food products in terms of potential contaminants such as aflatoxins.
5.1. Conclusions
The probiotic strains L. plantarum, L. paracasei, and L. casei isolated from Khiki cheese in this study demonstrated remarkable efficiency in binding AFM1 in both PBS and milk media. Given their potential to detoxify AFM1 in contaminated milk, they could be considered food-grade additives for reducing possible AFM1 contamination in dairy products. In this context, the isolated probiotics could be used as safe starter cultures for the production of commercial cheese and other dairy products. Moreover, these LAB strains, isolated from cheese, are environmentally friendly and capable of enhancing the health benefits of products. Therefore, the production of these strains on an industrial scale is recommended.
| Sample | Spiked AFM1; (ng mL-1) | AFM1 Reduction (%) |
|---|---|---|
| PBS + Lactobacillus paracasei | 50 | 71.35 |
| PBS + Lactobacillus casei | 50 | 53.5 |
| PBS + Lactobacillus plantarum | 50 | 62.5 |
| PBS | 50 | 0 |
| Milk + Lactobacillus paracasei | 50 | 68.53 |
| Milk + Lactobacillus casei | 50 | 57.20 |
| Milk + Lactobacillus plantarum | 50 | 66.42 |
| Milk | 50 | 0 |