1. Background
Root-end resection surgical procedures involve applying a root-end filling material to efficiently seal the area and allow the periradicular tissues to repair and regenerate (1). Retrograde filling materials, which remain in close contact with the tissues around the root apex for an extended period, should ideally be non-cytotoxic. Therefore, any material intended for use must be evaluated for its biocompatibility to ensure it is safe for the human body. These materials should not trigger inflammatory processes, as inflammatory cells release cytokines and other substances that cause bone and tooth resorption, potentially leading to treatment failure (2, 3).
Some effector cells involved in inflammatory processes include polymorphonuclear (PMN) leukocytes or polymorphonuclear neutrophils, macrophages, and T lymphocytes, which can destroy osteoclasts (4). Pro-inflammatory cytokines such as Interleukin 1 (IL-1β) and tumor necrosis factor (TNF-α) have been shown to play a crucial role in bone loss by stimulating apoptosis in osteoblasts or osteoblast precursors (5, 6). Additionally, osteoclastogenesis is regulated by three members of the TNF-α superfamily: Osteoprotegerin (OPG), receptor activator of nuclear factor-kappa B (RANK), and RANK-ligand (RANK-L). RANK-ligand is a member of the membrane-associated TNF-α ligand family and is expressed by osteoblasts, mesenchymal cells of the osteoblast lineage, stromal cells, fibroblasts, and activated T cells. It binds to RANK, which is expressed on the surface of preosteoclasts and osteoclasts, stimulating both the differentiation of osteoclast progenitors and the activity of mature osteoclasts. Osteoprotegerin acts as a decoy receptor that binds to RANK-L, preventing it from binding to RANK and thereby reducing osteoclast formation (7-9).
Numerous materials with various compositions have been proposed for use in retrograde fillings, such as amalgam, gutta-percha (GP), glass ionomer cement (GIC), composite resins, resin ionomer hybrids (e.g., Geristore), intermediate restorative material (IRM), gold foil pellets, Cavit, Diaket, Super ethoxy benzoic acid (EBA), and mineral trioxide aggregate (MTA) (10, 11). Geristore, a modified composite resin, is a hydrophilic, non-aqueous polyacid material that offers several advantages, including increased adhesion to tooth structure, insolubility in oral fluids, dual-cure capabilities (self-curing and light-curing), low curing shrinkage, a low thermal expansion coefficient, fluoride release, and radiopacity (12, 13).
Relatively few studies have investigated the biocompatibility aspects of Geristore. For instance, Bonson et al. assessed the effects of Geristore and MTA on the survival of periodontal ligament and gingival fibroblasts and on osteogenic gene expression. They also examined how these filling materials influenced cellular alkaline phosphatase (ALP) activity, an indicator of cementogenesis (14). Additionally, Gupta et al. evaluated cell adhesion and the in vitro biocompatibility of human periodontal fibroblast cells exposed to resin ionomer Geristore, MTA, and GIC using the MTT assay. They found that Geristore exhibited superior cell adhesion and less cytotoxicity to human periodontal ligament cells compared to MTA and GIC (12). Furthermore, the osteogenic response of MC3T3-E1 cells to Geristore and MTA was examined through MTT and ALP activity assays, revealing that Geristore was more effective in early cell proliferation and adhesion, while MTA showed superior ALP activity (15).
To the best of the authors’ knowledge, only one in vivo study (16) has investigated the effects of Geristore on the production of inflammatory cytokines.
2. Objectives
Therefore, the aim of the present study is to evaluate and compare Geristore with two more commonly used retrograde filling materials, ProRoot MTA and Super EBA, on the production of IL-1β, TNF-α, OPG, and RANK-L, as well as on the expression of RANK. Additionally, the study investigates the effect of these materials on cell survival.
3. Methods
3.1. Cell Culture
The human osteosarcoma cell line (MG-63) and the macrophage cell line (THP-1) were cultured in RPMI 1640 medium (Sigma, Steinhem, Germany) supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Grand Island, USA), 2 mM L-glutamine (Sigma, Steinhem, Germany), 100 µg/mL streptomycin, and 100 u/mL penicillin. The cultures were maintained in a humidified incubator at 37°C with 5% CO2.
3.2. Materials Preparation
The testing materials used were Super EBA (Bosworth Company, Skokie, IL, USA), ProRoot MTA (Dentsply, Tulsa, OK, USA), and Geristore (AngelusTM, Londrina, PR, Brazil). Three standard cylindrical blocks of each material, measuring 10 mm in diameter and 3 mm in height, were prepared under aseptic conditions according to the manufacturers' instructions. The cylindrical discs were allowed to set for 24 hours in a humidified atmosphere at 37°C.
3.3. MG-63 Co-culture with Filling Materials
MG-63 cells (106 cells/well) were cultured in the presence of three blocks each of MTA, Super EBA, Geristore, 107 IU/mL estradiol (Cayman Biomedical Company), and 108 IU/mL vitamin D3 (Alexis Biochemical) individually, in a humidified atmosphere at 37°C. Estradiol was used as a positive control for RANK-L release and a negative control for OPG release, while vitamin D3 was used as a positive control for OPG release and a negative control for RANK-L release. The supernatants of the cultures were collected after 24 and 48 hours for RANK-L and OPG production.
3.4. Monocyte Preparation and Co-culture
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll gradient centrifugation from buffy coats purchased from the Iranian Blood Transfusion Organization. The PBMCs were plated in tissue culture using RPMI 1640 medium (Sigma, Steinhem, Germany), supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Grand Island, USA), 2 mM L-glutamine (Sigma, Steinhem, Germany), 100 µg/mL streptomycin, and 100 u/mL penicillin. After a 1-hour plastic adherence step, non-adherent lymphocytes were gently washed away. Adherent blood monocytes and THP-1 cells were then cultured in RPMI 1640 medium along with cylindrical samples of MTA, Super EBA, Geristore, and lipopolysaccharide (LPS) (cat. no: L-6526, Sigma). Lipopolysaccharide was used as a positive control. After 24 hours, the supernatants of the cells were collected to analyze the concentrations of IL-1β and TNF-α.
3.5. Detection of Cytokines by ELISA
Cytokine production was detected in the supernatants of MG-63 cells (for measuring RANK-L and OPG) and in the culture supernatants of monocytes and THP-1 cells (for measuring IL-1β and TNF-α). The supernatants were collected and stored frozen at -20°C. Cytokine concentrations were measured using ELISA kits for IL-1β and TNF-α (R&D Systems Inc.), RANK-L (Immundiagnostik, Bensheim, Germany, and Apotech, Epalinges, Switzerland), and OPG (DRG System), according to the manufacturers' instructions.
3.6. Flow Cytometric Analysis
The RANK expression in monocytes was determined using the flow cytometry method. These cells were cultured with the supernatants of MG-63 cells that had been exposed to MTA, Super EBA, Geristore, estradiol (as a positive control), and vitamin D3 (as a negative control) for 24, 48, and 72 hours at 37°C. Monocytes were separated from the tissue culture plate using ice and 4% EDTA. The cells were then washed with PBS, stained with Mouse Anti-Human RANK antibody (Alexis Biomedical), and subsequently with FITC-anti-mouse IgG. The cells were analyzed using a FACS Analysis System (Becton-Dickinson).
3.7. MTT Assay
The cell viability of human monocytes exposed to Geristore, MTA, and EBA was analyzed using the MTT assay. Briefly, cells (104/well) were seeded in 96-well tissue culture plates containing the three filling materials and controls (LPS and medium without any material) for time intervals of 12 hours, 48 hours, and 120 hours. Tetrazolium bromide salt (10 μL per well in 100 μL of cell suspension) was added for 4 hours. At the end of the incubation period, the reaction mixture was carefully removed, and 100 μL of isopropanol containing 0.1% hydrochloric acid was added to each well. The plates were then read at 540 nm using a multi-well microplate reader.
3.8. Statistical Analysis
All data are presented as means ± standard deviation. Statistical analyses were performed using analysis of variance (ANOVA) with Minitab V17 software. A confidence level of 95% was considered for all tests. Post hoc pairwise comparisons were conducted using the Tukey test or the Games-Howell test, depending on the homogeneity of variance. Pearson correlation analyses were also conducted to determine whether there is a correlation between OPG and RANK-L, as well as between IL-1β and TNF-α. Bar charts were generated using GraphPad Prism (version 5).
4. Results
4.1. RANK-ligand and Osteoprotegerin Production
For the evaluation of RANK-L and OPG production, the human osteosarcoma cell line (MG-63) was cultured with filling materials for 24 and 48 hours. The values obtained from ELISA were statistically analyzed. The ANOVA results, shown in Appendix 1, indicated that the main effects of Material and Time were significant, with P-values of 0.011 and 0.007, respectively. However, their interaction effect was not significant (P-value = 0.800). Interaction is present when the dependent variable (i.e., concentration of RANK-L) at one factor level (e.g., Time) depends on the level(s) of another factor (e.g., Material).
The main effects of materials, as shown in Appendix 2 (left side), indicate that the lowest RANK-L concentration was associated with EBA, followed by MTA and Geristore, respectively. In contrast, Estradiol and Medium showed the highest concentrations of RANK-L. Additionally, the main plot for Time (Appendix 2, right side) demonstrates that the concentration of RANK-L was higher after 24 hours than after 48 hours. The Tukey pairwise comparisons revealed that the mean concentration of RANK-L for EBA after 48 hours was significantly lower than those for Estradiol and Medium after 24 hours. The mean values of RANK-L concentrations, their standard deviations, and the significant differences are shown in Figure 1. Means that do not share a letter are significantly different. Therefore, there were no significant differences between the filling materials after 24 and 48 hours of exposure.
The ANOVA results in Appendix 4 show that, similar to RANK-L, the main effects of material and time were significant for OPG. These main effects are illustrated in Figure 2, which indicates that Geristore produced the lowest OPG concentration. In contrast, the other materials resulted in much higher OPG concentrations. The main plot for time shows that as the duration increased from 24 to 48 hours, the concentration of OPG became higher. The mean OPG concentration produced by Geristore after 24 hours was significantly lower than those of the other materials after both 48 and 24 hours, except for VitD3. However, the mean OPG concentration generated by Geristore after 48 hours was significantly lower than that of MTA, Estradiol, and Medium. Additionally, there was no significant difference between Geristore and EBA in OPG concentration after 48 hours. Meanwhile, the difference in mean OPG concentrations produced by MTA, EBA, and the control groups was not statistically significant after 24 hours.
4.2. IL-1β and Tumor Necrosis Factor Production
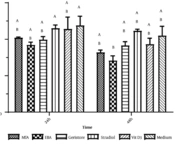
Concentrations of IL-1β and TNF-α were obtained from the culture supernatants of both THP-1 cells and monocytes. These concentrations were statistically analyzed using Minitab software. Welch’s test showed that the material used was a significant factor (P-value = 0.006) influencing TNF-α concentration (Figure 3). This figure shows that Geristore caused lower TNF-α production compared to MTA and EBA. Furthermore, the Games-Howell pairwise test was conducted and found significant differences between LPS (as the positive control group) and the other filling materials. However, the differences between the other groups were not significant.
The ANOVA results indicated that the material used was a significant factor in determining IL-1β concentration (Figure 3). Ethoxy benzoic acid resulted in the lowest IL-1β production, followed by Geristore and MTA. Additionally, the Tukey pairwise test was conducted to identify significant differences between the groups (i.e., filling materials). Figure 3 shows the mean values of IL-1β concentrations, the standard deviations, and the significance between the groups. Lipopolysaccharide and EBA were significantly different from each other and from the other filling materials. Furthermore, the mean IL-1β concentration caused by Geristore was significantly lower than that caused by Medium. However, the differences between Geristore and MTA, as well as between Medium and MTA, were not significant.
Peripheral blood mononuclear cells derived from five donors were cultured with three retrograde filling materials, and after 24 hours, their supernatants were collected and evaluated for IL-1β and TNF-α production by ELISA. Figure 4 shows the bar charts of the actual and transformed values. Welch’s test indicated P-values of 0.030 and 0.001 for IL-1β and TNF-α concentrations, respectively, suggesting that the type of material is a significant factor influencing these concentrations. Furthermore, the Games-Howell pairwise test was used. A significant difference was found between Geristore (higher) and EBA (lower) in IL-1β concentrations (Figure 4). Regarding TNF-α concentrations, there was no significant difference between Geristore and EBA or between Geristore and MTA (Figure 4). However, the mean TNF-α concentration caused by MTA was significantly higher than that caused by EBA.
The Pearson analysis of the concentrations of TNF-α and IL-1β showed that the correlation between these two cytokines was 0.885 (P-value = 0.001) in THP-1 cells. The correlation between the transformed concentrations of TNF-α and IL-1β was 0.502 (P-value = 0.057) in monocytes. The high correlation coefficients indicate a strong relationship between TNF-α and IL-1β. The positive value shows that the changes in the concentrations of the two cytokines are in the same direction.
4.3. Receptor Activator of Nuclear Factor-Kappa B Expression
The RANK expression of monocytes cultured with MTA, EBA, Geristore, estradiol, and vitamin D3 for 24, 48, and 72 hours is shown in Figure 5. In monocytes exposed to estradiol and MTA, RANK expression was upregulated compared to the other groups. The graph of the flow cytometry results is presented in Appendix 5.
4.4. Cell Survival
The MTT assay was conducted at different time points to examine the effect of the materials on the survival of PBMCs. The ANOVA results, shown in Appendix 6, indicate that the main effects of Material and Time, as well as their interaction effects (material*time), were significant. Appendix 7 illustrates the interaction effects of material*time. This figure shows a clear interaction (as the lines are not parallel in the plot), suggesting that the effect of the filling materials on OD depends on the exposure time. For example, the OD associated with EBA and MTA increased as the exposure time decreased. However, for Geristore and LPS, the highest OD was observed with 48 hours of exposure.
The Tukey test was also performed to determine whether the differences in OD among the materials, times, and their interactions were significant. Table 1 presents the significant differences between the different groups. It can be seen that the mean OD for Geristore after 48 hours of exposure was significantly higher than for Geristore after 12 hours and 120 hours of exposure, and EBA after 120 hours of exposure.
| Materials × Time | N | Means (Transformed) | Grouping |
|---|---|---|---|
| LPS 48h | 3 | 1.395 | A |
| Geristore 48h | 3 | 1.395 | A |
| MTA 12h | 3 | 0.969 | A, B |
| MEDIUM 12h | 3 | 0.947 | A, B |
| LPS 12h | 3 | 0.818 | A, B |
| LPS 120h | 2 | 0.511 | A, B, C |
| MTA 120h | 2 | 0.476 | A, B, C |
| MTA 48h | 3 | 0.463 | A, B, C |
| EBA 12h | 3 | 0.443 | A, B, C |
| MEDIUM 120h | 2 | 0.397 | A, B, C |
| MEDIUM 48h | 3 | 0.377 | A, B, C |
| EBA 48h | 3 | 0.338 | A, B, C |
| Geristore 12h | 3 | 0.159 | B, C |
| EBA 120h | 2 | 0.126 | B, C |
| Geristore 120h | 2 | 0.097 | C |
5. Discussion
The ideal root-end filling materials should be biocompatible and adhere well to the root canal wall (17). The purpose of this study was to assess the biocompatibility of three root-end filling materials: Mineral trioxide aggregate, Super-EBA, and Geristore. Mineral trioxide aggregate is a common root-end filling material composed of tricalcium silicate, tricalcium aluminate, tricalcium oxide, and silicate oxide, and it is used for pulp capping, pulpotomy, and repairing root perforations (18). Ethoxy benzoic acid was introduced in the 1960s as a substitute for zinc oxide-eugenol cements. This material has superior strength due to the ethoxy benzoic acid in its chemical formulation. However, it has been reported that EBA can cause a moderate cytotoxic reaction because of the eugenol component (19-21). Geristore, a resin ionomer, is a nonaqueous, hydrophilic, polyacid-modified composite resin with advantages such as insolubility in oral fluids and good adhesion to tooth structures (13). A study comparing the effects of Geristore with MTA and GIC on human periodontal fibroblasts revealed that Geristore was significantly better and less cytotoxic than MTA and GIC (11). Due to these superior material properties, this study evaluated the effect of these filling materials on the production of pro-inflammatory and resorptive cytokines.
Pro-inflammatory cytokines such as IL-1β and TNF-α play crucial roles in the host's antimicrobial and anti-tumor responses, but the production of these cytokines during inflammatory processes can stimulate bone resorption. In this study, the effects of the three types of root-end filling agents on pro-inflammatory cytokine production were investigated using human normal monocytes and the macrophage cell line (THP-1) after 24 hours of exposure. Exposure of monocytes to EBA for 24 hours resulted in decreased TNF-α and IL-1β secretion compared to the other groups. In THP-1 cultures, the release of pro-inflammatory cytokines did not differ significantly between the groups. However, Geristore caused the lowest TNF-α production, and for IL-1β secretion, it was second to EBA. In a study where MG-63 cells were cultured with MTA, RT-PCR results showed that the expression of IL-1β, IL-6, and IL-8 was detected in all samples with minimal TNF-α expression (22). This aligns with the results obtained here from co-culture with THP-1. Conversely, the culture of human dental pulp cells with MTA decreased the expression of IL-1β and IL-6 (23). In a study by Oliveira et al., where the calvarial bone of mice was exposed to MTA, Geristore, and Emdogain in vivo, MTA did not alter TNF-α expression but increased IL-6 expression. On the other hand, Geristore significantly down-regulated TNF-α, IL-6, TGF-β, and IL-4 mRNA (16).
The RANK-L/OPG pathway regulates the bone microenvironment. RANK-L, expressed in osteoblasts, promotes the differentiation of osteoclasts and inhibits their apoptosis, while OPG competes with RANK-L by binding to it, preventing the binding of RANK-L to RANK, which results in the inhibition of osteoclast differentiation and function. Therefore, the RANK-L/OPG pathway plays a crucial role in maintaining the balance between bone formation and resorption (24).
In our study, Geristore induced the highest level of RANK-L and the lowest level of OPG in cultures with the MG-63 cell line. A study by Hashiguchi et al. revealed that an MTA solution inhibited the 1α, 25(OH)₂D₃-induced downregulation of OPG mRNA and protein production but did not affect RANK-L-induced osteoclastogenesis in co-cultures of BMCs and POBs derived from OPG-deficient mice. Our findings also showed that MTA caused a moderate level of RANK-L production (between Geristore and EBA). Furthermore, Super-EBA solutions suppressed osteoclast formation in co-cultures by inhibiting proliferation (25). A study by Oliveira et al. showed that MTA promotes a significant increase in the mRNA expression of RANK-L, RANK, and OPG, while Geristore did not alter the basal expression of these mediators during the same evaluation period (16).
Monocytes are a subset of circulating white blood cells that mobilize and migrate to sites where they are needed. They play a crucial role in provoking immune responses during infections and driving inflammatory conditions (26). The MTT test results showed that Geristore produced the lowest viability both early (12 hours) and late (120 hours) compared to EBA and MTA when co-cultured with monocytes. Specifically, the order of materials in terms of viability at both early and late time points was Geristore < EBA < MTA. However, at 48 hours, EBA resulted in the lowest viability, followed by MTA and then Geristore.
In one study, the histopathologic effects of three materials—mineral trioxide aggregate (GMTA), Retroplast, and Geristore—were assessed by implanting them into the subcutaneous connective tissue of rats. The statistical results showed that the infiltration of inflammatory cells was significant for Geristore but not for GMTA, and Geristore also caused more necrosis (23). Histological evaluation of dorsal connective tissue in rats indicated that Geristore gradually increased chronic inflammation at the tube ends over the three test periods, with mild inflammation at one week and mild to moderate inflammation in the subsequent periods, leading to focal necrosis (27).
Overall, our results suggest that Geristore does indeed cause higher expression of RANK-L, which stimulates the fusion, deposition, activation, and survival of osteoclasts, leading to bone resorption. Additionally, Geristore performed poorly in the production of inflammatory cytokines. This could be related to the high viability and reduced death of monocytes it causes in the medium term (12 hours, 120 hours) compared to the other materials. Higher viability of these cells likely leads to greater secretion of inflammatory cytokines (after 24 hours). However, given the results obtained here and those from the literature, further studies are needed before definitive conclusions can be drawn.