1. Background
Many microorganisms cause diseases that are commonly treated with antibiotics. However, it is important to note that the effective period of each antibiotic is limited, after which microorganisms can develop resistance, rendering the antibiotic ineffective. Therefore, microbiologists are always searching for new antimicrobial agents. One approach to finding new drugs is to explore the natural sources of these drugs, such as plants. It is known that certain plants were used as remedies before microbes were discovered (1). Human beings have always utilized plants and their products as drugs since the beginning of human civilization. Medicinal herbs have significant economic value worldwide (2). Additionally, plants are much cheaper and more accessible than existing drugs. There are many herbs that could be used as drug sources, but they remain unknown. Among the many plant species, only a few percent have been studied phytochemically, with most still unexplored (3). Iran's vegetation is very diverse, and for many years, botanists have been working to collect and identify plants in different parts of Iran, studying them phytochemically and pharmacologically.
Within the legume family, there is a large genus called Astragalus L., which is dispersed across a wide range of temperate areas worldwide, primarily in North America, Europe, and Asia. About 2000 species have been reported, with 372 in North America and 133 in Europe (4). Astragalus has shown a variety of potential pharmaceutical applications in treating immunodeficiency syndromes, cancer, and regulating heart and kidney functions. The roots of Astragalus are used to improve immune function and increase stamina strength. Astragalus is also used to treat respiratory diseases and strengthen respiratory health (5). Other traditional therapies use Astragalus to treat sweating, persistent injuries, paralysis, and swelling (6).
2. Objectives
In this research, we collected, dried, and identified five species of Astragalus from mountainous areas in some parts of southeastern Iran in Kerman Province. The methanolic extracts of these species were then prepared, tested, and compared with each other using the antibiogram test for antimicrobial activity.
3. Methods
3.1. Collection of Plant Material
Fresh plants from the mountainous region (Takht-e-Sartashtak) of southeastern Iran in Kerman Province, with an average height of 3600 meters, were collected, dried, and identified in the spring and summer of 2017 and 2018. The whole parts of dried plants were used for extraction.
3.2. Preparation of Plant Extracts
For the extraction of the dried plants, 10 g of each powdered plant was mixed with 50 mL of methanol solvent. The mixture was then placed on a shaker for 3 days to isolate the methanol-soluble compounds from the plant and dissolve them in the solvent. The mixture was then filtered with filter paper, and the extract was left to dry. The resulting powder was ready for antimicrobial work (7).
3.3. Tested Microbes
The Knowledge Base company of Dana Gene Researchers, Kerman, Iran, provided the standard microbial species. The bacterial species tested included one fungus, Candida albicans (PTCC:5027), two gram-negative bacteria, Pseudomonas aeruginosa (PTCC:1074) and Escherichia coli (PTCC:1330), and two gram-positive bacteria, Staphylococcus aureus (PTCC:1112) and Bacillus cereus (PTCC:1015) (8).
3.4. Evaluation of Antimicrobial Activity
First, the dried extract of the plant was used to prepare different dilutions (mg/mL). Then, 0.05 g of the dry extract of the plant was weighed with a digital scale and dissolved in 1 mL of Dimethyl sulfoxide (DMSO) solvent to obtain an extract with a dilution of 50 mg/mL. Further dilutions were similarly prepared. The Mueller Hinton Agar culture medium, manufactured by Merck in Germany, was used to evaluate the effect of the plant extract on selected microorganisms. After preparing the culture medium, 0.5 McFarland of microorganisms was prepared in sterile physiological serum, and 0.2 µL of the suspension was poured onto the culture medium under aseptic conditions. The microorganism was then cultured on Mueller Hinton Agar using a glass spreader to achieve complete uniformity. In the next phase, wells were created in each culture medium at regular intervals, and the 50 mg/mL extract dilution was poured into the wells (9). The amount of extract in each well was 20 μL, added using a sampler. The plates were incubated at 30°C for 24 hours to test and check the growth inhibition zone.
It should be noted that a well containing negative control DMSO was used, and for a positive control, a commercially available antibiotic disk was employed (10).
3.5. Characterization of Minimum Fungicidal Concentration, Minimum Bactericidal Concentration, and Minimum Inhibitory Concentration
To determine the Minimum Fungicidal Concentration (MFC), minimum bactericidal concentration (MBC), and minimum inhibitory concentration (MIC), we used the broth dilution method (9). First, the preparation of the standard solution was done as follows: 0.5 mL of 1% dehydrated barium chloride solution was added to a 100 mL dish, and the volume was increased to 100 mL with 1% sulfuric acid. The resulting barium sulfate solution is called the 0.5 McFarland standard opacity solution. To obtain a dilution of 50 mg/mL of the extract, 50 mg of plant extract powder was poured into 1 mL of solvent. In each of the 5 test tubes, 1 mL of the extract solution was poured, and 1 mL of the standard opacity solution was added to each tube. The results were considered after incubating the tubes for 18 hours. The last tube without microorganism growth or opacity was considered the MIC, and the previous tube was usually the MBC or MFC. As a rule, the contents of the MBC and MFC tubes should not show any growth on the culture medium (11).
4. Results
The methanolic extracts of five Astragalus species were examined for their antimicrobial activity against one fungus, two gram-negative bacteria, and two gram-positive bacteria using Mueller Hinton Agar culture medium and the agar-well diffusion method.
The results showed that the methanolic extracts of four of these plant species were active against at least three microorganisms. One species, Astragalus siliquosus, did not show any antimicrobial activity. Table 1 presents the results of the antimicrobial activity of the five plants against the five microorganisms mentioned.
| Plants Name | Escherichia coli | Staphylococcus aureus | Bacillus cereus | Pseudomonas aeruginosa | Candida albicans |
|---|---|---|---|---|---|
| Astragaluscemerinus Beck | - | 13 | 13 | 11 | - |
| Astragalusdactylocarpus Boiss. | - | 13 | 10 | - | 10 |
| Astragalus glaucacanthosFisch. | 10 | 12 | 12 | 12 | - |
| Astragalusrhodosemius Boiss. and Hausskn. | - | 14 | 10 | 11 | - |
| Astragalussiliquosus Boiss. | - | - | - | - | - |
a Zone of growth inhibition (mm, ± 1).
It was found that the plant species displayed different antimicrobial activities. Astragalus glaucacanthus was active against four of the five microorganisms and was the only plant active against Escherichia coli. Astragalus siliquosus did not show any antimicrobial activity against any of the microorganisms. The greatest antimicrobial activity was observed with Astragalus rhodosemius against Staphylococcus aureus. The values of the diameter of the growth inhibition zones for the five species are shown in Table 1. The MIC, MBC, and MFC of the plant extracts were approximately similar (Table 2). The total values of MIC, MBC, and MFC of the plant extracts are shown in Table 2.
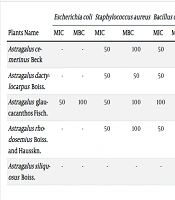
| Plants Name | Escherichia coli | Staphylococcus aureus | Bacillus cereus | Pseudomonas aeruginosa | Candida albicans | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MFC | |
| Astragaluscemerinus Beck | - | - | 50 | 100 | 50 | 100 | 50 | 100 | - | - |
| Astragalusdactylocarpus Boiss. | - | - | 50 | 50 | 50 | 100 | - | - | 50 | 100 |
| Astragalus glaucacanthosFisch. | 50 | 100 | 50 | 100 | 100 | 100 | 50 | 100 | - | - |
| Astragalusrhodosemius Boiss. and Hausskn. | - | - | 50 | 100 | 50 | 50 | 50 | 100 | - | - |
| Astragalussiliquosus Boiss. | - | - | - | - | - | - | - | - | - | - |
Abbreviations: MBC, minimum bactericidal concentration; MFC, minimum fungicidal concentration; MIC, minimum inhibitory concentration.
a MIC, MBC and MFC of plants extracts against 5 microorganisms (mg/mL, ± 1).
5. Discussion
So far, many studies have been conducted worldwide to investigate the medicinal and antimicrobial properties of different species of Astragalus. In most cases, these studies have shown positive effects on various diseases as well as their antimicrobial activity. Many Astragalus species are used as medicinal plants; for example, one of the most widely used and studied species is Astragalus membranaceus, which is officially listed in the Chinese Pharmacopoeia, like the species Astragalus mongholicus (Astragalus membranaceus var. mongholicus) and Astragalus complanatus. Some medicines are made from extracts of these species. The pharmacological activity of Astragalus is attributed to two chemical compounds: Polysaccharides and saponins. Most of the potential therapeutic effects include liver protection, immune stimulation, and antiviral activity (12).
The effect of extracts from some wild Egyptian Astragalus species was studied for their hypoglycemic effects on alloxanized diabetic rats (13). The saponin astramembrannin I in Astragalus prevented the increase in vascular permeability caused by serotonin and histamine and decreased edema in rats (14). The antimutagenic activity of Astragalus membranaceus aqueous extract on aflatoxin B1-induced mutagenesis, using Salmonella typhimurium as a test strain and rat liver ultra-supernatant as the activating system, has been demonstrated (15). There are also positive results for using this medicinal plant as a chemopreventive agent for cancer in China. Some medicinal herbs from China are known to stimulate sperm motility (16), and Astragalus membranaceus aqueous extract was found to be active in this regard. However, it is noteworthy that some constituents of Astragalus species can negatively affect male reproduction (17). Therefore, Astragalus species are a group of medicinal plants whose active ingredients are primarily saponins and polysaccharides (12).
The aqueous root extracts of some Astragalus species are used to promote wound healing and treat leukemia in folk medicine in Turkey (18, 19). Many compounds found in the polysaccharide- and saponin-rich roots of Astragalus species have effects on the treatment of cancer and the effective functioning of the immune system (12, 19-21). Astragalus is one of the largest herbal genera, with many species. In Iran, this genus also has several species that can be used effectively by collecting, identifying, and evaluating their properties and developing new drugs for treating diseases.
Previous research has shown that Astragalus is a traditional medicinal plant used in many countries, including China, for treating various diseases, including anemia, diabetes, digestive diseases, nervous and respiratory system diseases, and some types of cancer. Its role in preventing bone loss and its anti-aging properties have also been proven (22).
Despite the proven antimicrobial activity of many plants, these plants are still not widely used in medical science. Therefore, pharmacists and doctors are working to enhance the status of these plants in medical science (23).