1. Background
Multiple sclerosis (MS) is an inflammatory autoimmune disease that destroys myelin and neurons in the central nervous system (CNS). It is believed that the spontaneous reaction of T lymphocytes in the CNS leads to damage to oligodendrocytes, resulting in damage to the myelin sheath. Inflammatory responses in the white matter cause lesions that lead to severe physical disabilities (1).
Reactive oxygen species (ROS) mediate myelin sheath destruction and axonal damage. The local level of ROS increases during inflammation, disrupting the antioxidant defense potential in nerve lesions. ROS can damage proteins, lipids, and nucleic acids, leading to cell death (2, 3).
Due to the complexity of MS etiology, it is very difficult to find an animal model that exactly mimics the conditions and symptoms of MS patients. Therefore, different models are used to investigate various aspects of the disease. The pathological hallmarks of MS are white matter demyelination, inflammation, axon damage, and blood-brain barrier (BBB) disruption. To investigate demyelination in white matter and axonal damage (including the corpus callosum of the brain), models that cause chemical damage in the brain, such as cuprizone, lysolcitin, and ethidium bromide, are usually used. These models are valuable for studying the mechanisms of demyelination and remyelination, as remyelination is initiated upon the termination of the chemical injury reagent (4-7). Various preclinical models with different specific features of the disease are available to study MS pathogenesis and to develop new therapeutic options. Over the last decade, the toxic demyelination model induced by cuprizone has become increasingly popular and has contributed significantly to our understanding of distinct yet important aspects of MS pathology.
Previous research has shown that natural products and Iranian medicine have fewer side effects and are more effective in treating chronic diseases (8). Boswellia is one such natural product obtained in the form of resin from the trees Boswellia carterii Birdw and Boswellia bhaurdajiana Birdw, which belong to the Oleaceae family. Boswellia serrata is indigenous to northwest India and contains six main compounds, including alpha and beta boswellic acids, with acetyl-11-keto-β-boswellic acid (AKBA) being one of its most important natural compounds (9-11).
Recent research has shown that frankincense and its active ingredients have neuroprotective properties and can create new neural networks in the brain. Due to the disruption and disconnection of nerve networks in neurodegenerative diseases such as MS, which leads to the loss of cognitive abilities and memory, Boswellia serrata (frankincense) seems to be a promising treatment strategy (12, 13).
Acetyl-11-keto-β-boswellic acid is a pentacyclic triterpenoid compound and one of the most important active components in the multicomponent mixture of Boswellia serrata resin. Extracts of Boswellia serrata resin (boswellic acids) have shown in vivo antioxidant activity in many conditions, including intestinal disease, myocardial I/R injury, and pulmonary fibrosis. The neuroprotective properties of pentacyclic triterpenoids have recently attracted more attention (11).
Recently, it has been reported that ursolic acid, a natural pentacyclic triterpenoid, enhances neuroprotection after cerebral ischemia in mice by activating the Nrf2 pathway. Another study demonstrated that AKBA may have a superior antioxidant effect compared to ursolic acid in mice. Therefore, we hypothesized that AKBA, which has a similar chemical structure to ursolic acid, may promote neuroprotection and reduce oxidative stress and neuroinflammation through activation of the Nrf2 pathway (14).
Studies have proven that boswellic acids inhibit the production of pro-inflammatory enzymes, such as 5-lipoxygenase and leukotriene B4, and reduce lipid peroxidation and oxidative stress (15, 16). Previous reports have shown that Boswellia serrata extract reduces brain damage, neuronal cell apoptosis, and the severity of lipid peroxidation. It also increases glutathione capacity and superoxide dismutase activity in the cerebral cortex following a stroke (16). Additionally, it promotes axonal growth and the formation of new neuronal branches, which increases the length and number of dendrites. It also protects against memory loss caused by hypothyroidism (15, 17, 18).
Frankincense extracts from different species have been found to possess anti-inflammatory properties that can help with a range of medical conditions. These include rheumatoid arthritis, ulcerative colitis, bronchitis, sinusitis, and irritable bowel syndrome. Furthermore, frankincense extracts can reduce the risk of asthma and have been shown to activate genes involved in tumor cell apoptosis. As a result, they possess antitumor effects and can help with diseases such as bladder carcinoma, leukemia, and glioblastoma (11, 19).
Many studies have found that AKBA has anti-inflammatory, antioxidant, and anticancer effects. It prevents obsessive-compulsive disorder, protects against acute spinal cord injury and damage to human lens epithelial cells in cataracts, promotes liver regeneration and recovery in diabetic mice, and protects against learning and memory disorders caused by lipopolysaccharides. Additionally, it reduces oxidative stress and profibrotic mechanisms and improves vascular regeneration and spontaneous hypertension (19-26). Acetyl-11-keto-beta-boswellic Acid activates the ERK pathway and promotes Schwann cell proliferation to accelerate the healing of sciatic nerve injury (24).
2. Objectives
Given the strong anti-inflammatory and antioxidant effects of frankincense and its active ingredient, acetyl-11-keto-beta boswellic acid, and the lack of research on its histopathological effects and oxidative stress (particularly on the myelin sheath) in the MS disease model, this study investigates the potential protective effects of AKBA on the brain in the cuprizone-induced MS model.
3. Methods
3.1. Animals and Study Design
Thirty-two adult female C57/BL6 mice (WT; 20 - 25 g, 8 - 10 weeks old) were purchased from the Karaj Pasteur Institute. The mice were housed under standard conditions, including 12-hour light/dark cycles, consistent humidity (40 - 70%), and a controlled temperature (22 ± 2°C), with ad libitum access to water and food. All experimental procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Ethics Committee of Semnan University of Medical Sciences (Approval ID: IR.SEMUMS.REC.1396.253). The animals were randomly divided into four groups of eight as follows:
(1) Control group (CO): The animals in this group were fed a normal diet for 6 weeks and received an intraperitoneal saline solution (as a carrier for cuprizone).
(2) Cuprizone-treated group (CPZ, MS disease model): The animals in this group received food containing 0.3% cuprizone for 6 weeks.
(3) Pre-treatment group (AKBA + CPZ): Before receiving cuprizone (0.3%), the animals in this group were administered 11-keto-beta boswellic acid at a dose of 50 mg/kg intraperitoneally every other day for 4 weeks.
(4) Post-treatment group (CPZ + AKBA): After receiving cuprizone (0.3%), the animals in this group were given 11-keto-beta boswellic acid intraperitoneally at a dose of 50 mg/kg every other day for 4 weeks.
Twenty-four hours after the end of the study, the animals were euthanized under a high dose of anesthesia, and brain sampling was performed for histopathological, biochemical, and immunohistochemical investigations.
3.2. Induction of Cuprizone-demyelination Model
In this study, the cuprizone-induced demyelination model developed by Morel and colleagues in 1998 was used to induce extensive demyelination similar to that seen in MS. The neurotoxic substance cuprizone was added to the mice's food at a concentration of 0.3%, and the mice consumed it orally for six weeks. To prepare the cuprizone-containing food, the rodent food pellets were ground into a powder. Cuprizone was then added at a dose of 0.3% and mixed uniformly. Normal saline was added to this homogeneous mixture to form a paste. Finally, the paste was shaped into food pellets for the animals using a 2.5 cc syringe and allowed to dry. The cuprizone-containing food was provided to the animals 5 days a week for six weeks (9, 10). The rotarod behavioral test and Luxol fast blue staining were used to detect demyelination and confirm the experimental model of MS (27, 28).
3.3. Preparation and how to use Acetyl-11-keto-beta-boswellic Acid
Acetyl-11-keto-β-boswellic acid (90% purity, Santa Ana, CA) was first diluted with saline and administered by intraperitoneal injection. In groups 4 and 5, AKBA was injected intraperitoneally at a dose of 50 mg/kg, with an injection volume of 2 mL/kg. In the sham (vehicle) group, only 2 mL/kg of saline was injected. The dose of 50 mg/kg AKBA administered to the mice (corresponding to approximately 350 mg of Boswellia serrata extract per kg) was selected based on previous studies (23, 29).
3.4. Evaluation of Rotarod Behavioral Test
To diagnose demyelination and assess the experimental model of MS, we evaluated the animals' movement balance using the rotarod behavioral test. This test was conducted twice a day, in the morning and evening, for three consecutive days at the end of the study. The rotarod test followed the procedure outlined by Lundblad et al. The rotarod device (model 865) consists of a rotating rod that increases in speed over time. The rotation speed started at 5 rotations per minute and gradually increased to a maximum of 40 rotations per minute within 120 seconds, maintaining the maximum speed for the remainder of the test.
To evaluate the animals' balance and coordination, they were placed on the rotating rod at an initial speed of 5 rotations per minute, and the timer was started. The rotation speed continued to increase until it reached 40 rotations per minute. When an animal lost its balance and fell from the rod, the timer was stopped, and the time it maintained balance on the device was recorded. It is important to note that the animals underwent training before the main test (30, 31). During the final days, the animals' movement coordination and balance were evaluated across all groups and compared.
3.5. Histopathological and Immunohistochemical Evaluation
At the end of the study, to evaluate the histopathology and immunohistochemistry of the brain tissue, all mice were deeply anesthetized with 100 mg/kg of pentobarbital and perfused transcardially via the ascending aorta with 0.1 M phosphate-buffered saline (PBS, pH = 7.4), followed by 4% paraformaldehyde in PBS. The brains were then removed and fixed in 2.5% paraformaldehyde for 48 hours. Serial coronal sections (6 - 8 μm thickness) were cut using a rotary microtome (Leica Biosystems, USA) and placed on slides.
To study the extent of myelin destruction in the corpus callosum area, specific myelin staining was performed using Luxol Fast Blue 1% (LFB). This staining method is typically used to identify the myelin sheath in the central nervous system and to evaluate axon repair and regeneration (remyelination and demyelination). In this method, nerve bundles rich in myelin, such as the corpus callosum, appear light blue to turquoise blue, while the brain cortex exhibits a dull color.
For immunohistochemical staining, free-floating sections were first pre-treated with 5% hydrogen peroxide in methanol for 20 minutes, followed by washing with Tris solution (pH 7.4) three times (3 minutes each). The slides were then placed in citrate buffer solution (pH 6) at 98°C for 10 to 15 minutes to retrieve tissue antigens. After washing with TBS, the sections were incubated for 2 hours at room temperature (22 - 24°C) in a blocking solution composed of 1% Triton X-100 and 5% normal goat serum with 1% BSA in TBS. The sections were then incubated in the blocking solution for 48 hours at 4°C with the primary antibody for oligodendrocytes (Anti-Olig2 antibody, 1:50, ab254043). After rinsing in TBS buffer three times (5 minutes each) with gentle shaking, the brain sections were incubated with the diluted secondary antibody (Anti-Rabbit 2013-07 Dako HRP) in TBS solution with 1% BSA at a dilution of 1:500 for 2 hours at room temperature. Following additional TBS rinses, the slices were incubated with Streptavidin-Peroxidase Polymer (Ultrasensitive S 2438-sigma) in TBS solution at a dilution of 1:200 for 30 minutes at room temperature. The antigen–antibody complexes were then visualized using 1% 3,3′-diaminobenzidine (DAB, Sigma-Aldrich, St. Louis, MO, USA) as the chromogen. Immunohistochemical controls were performed using the same method, but without the primary antibodies. After dehydration and clarification, the slices were mounted with a cover slip and Entellan mounting medium (32, 33).
3.6. Interpreting the Results of Luxol Fast Blue Staining and Immunohistochemistry
In this study, Luxol Fast Blue staining was used to investigate the degree of myelin sheath destruction, and the immunohistochemical method was employed to count oligodendrocyte cells in the corpus callosum area of the brain at Bregma (0.2 mm to -1.4 mm). This area is located next to the lateral ventricle, making it easier to identify. The density of myelinated axons in this region is significantly higher than in adjacent areas, allowing for a more precise study of the myelin sheath. Additionally, to count mature oligodendrocyte cells, the entire corpus callosum area was examined.
Photographs of the stained sections were taken using a digital camera (Labomed, USA). The areas of blue myelin sheaths, empty spaces (demyelinated), and the number of oligo-2 positive cells (stained brown) in the corpus callosum were evaluated using Image J Software (Soft Imaging System, Berlin, Germany) and light microscopy (Olympus, Tokyo, Japan) at ×100 and × 400 magnifications. The number of oligo-2 positive cells in the corpus callosum of the mice brain (five sections per mouse) was counted and expressed as a number per square millimeter.
3.7. Malondialdehyde (MDA) and Total Antioxidant Capacity (TAC) Assay
The MDA levels were assessed in brain tissue samples isolated from rats using a commercially available MDA assay kit (Cat no. S0131; Beyotime Institute of Biotechnology, Haimen, China), following the manufacturer's protocol. Briefly, brain tissue sections were homogenized in 0.15 ml of thiobarbituric acid (TBA) diluent, 0.05 ml of TBA, and 3 µL of antioxidant. The mixture was then heated and boiled for 15 minutes. After centrifugation at 1000 × g for 10 minutes at 4°C, the supernatants were collected, and the absorbance of the samples was measured at 532 nm using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). MDA levels were expressed as µmol/mg protein (34).
To measure the total antioxidant capacity (TAC), the animals' brains were cold-washed with a 0.9% saline buffer and preserved in a freezer at -80°C for long-term storage. The brains were initially homogenized using a homogenizer and sonicator device. The tissues were then centrifuged at 1000 × g for 10 minutes at 4°C, and the supernatant was separated and used to measure oxidative stress markers via spectrophotometry using specific kits. Total antioxidant capacity measurement was conducted based on the method described by Benzie and Strain (1999) (16, 35).
3.8. Statistical Analysis
Statistical analysis was performed using SPSS version 22.0 (Statistical Product and Service Solutions Inc.). A one-way analysis of variance (ANOVA), followed by Tukey's post hoc test, was used for statistical evaluation. All data are presented as mean ± SEM, with the significance level set at P < 0.05.
4. Results
4.1. The Effects of Acetyl-11-keto-beta-boswellic Acid on Animals' Weight
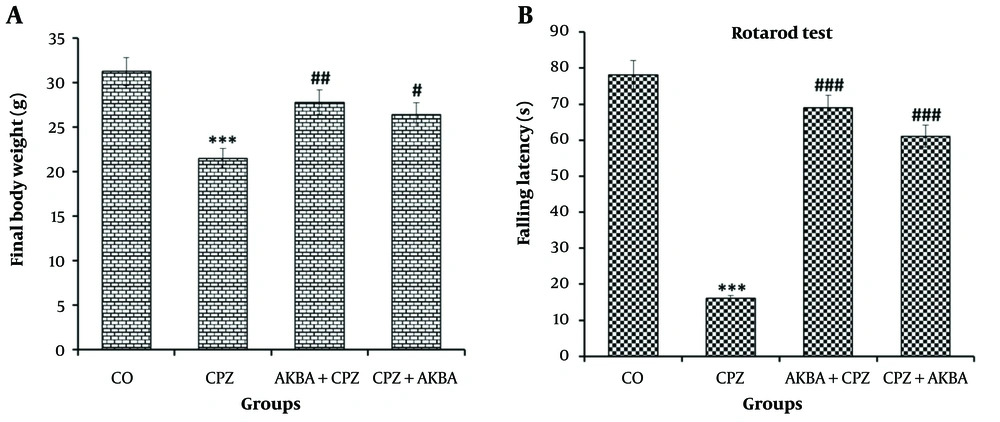
Data on animal weight (AW) are shown in Figure 1A. The comparison between the groups showed that AW in the CPZ group decreased significantly compared to the control group (P < 0.001). Acetyl-11-keto-beta-boswellic Acid administration improved the reduction in AW in the AKBA + CPZ (P < 0.01) and CPZ + AKBA (P < 0.05) groups compared to the CPZ group.
4.2. The Effects of Acetyl-11-keto-beta-boswellic Acid on Rotarod Behavioral Test
Balance duration (BT) data on the rotarod device are shown in Figure 1B. The comparison between the groups showed that BT in the CPZ group decreased significantly compared to the control group (P < 0.001). Balance duration was significantly increased in the AKBA + CPZ and CPZ + AKBA groups compared to the CPZ group (P < 0.001). In summary, the findings showed that the administration of AKBA (both pre- and post-treatment) improved the animals' balance time, which was disrupted by cuprizone-induced multiple sclerosis. Pre-treatment administration of AKBA showed a more significant effect than post-treatment.
The effect of acetyl-11-keto-beta boswellic acid on body weight at the time of sampling (A); and balance time in the rotarod test (B); in an animal model of cuprizone-induced demyelination. Data are presented as Mean ± SEM. *** represent P < 0.001 vs. the control group; #, ## and ### represent P < 0.05, P < 0.01 and P < 0.001 vs. the CPZ. CO: Control; CPZ: Cuprizone-induced demyelination model; AKBA + CPZ: CPZ group pre-treated with acetyl-11-keto-beta boswellic acid; CPZ + AKBA: CPZ group post-treated with acetyl-11-keto-beta boswellic acid.
4.3. The Effects of Acetyl-11-keto-beta-boswellic Acid on the Demyelination Area of the Corpus Callosum
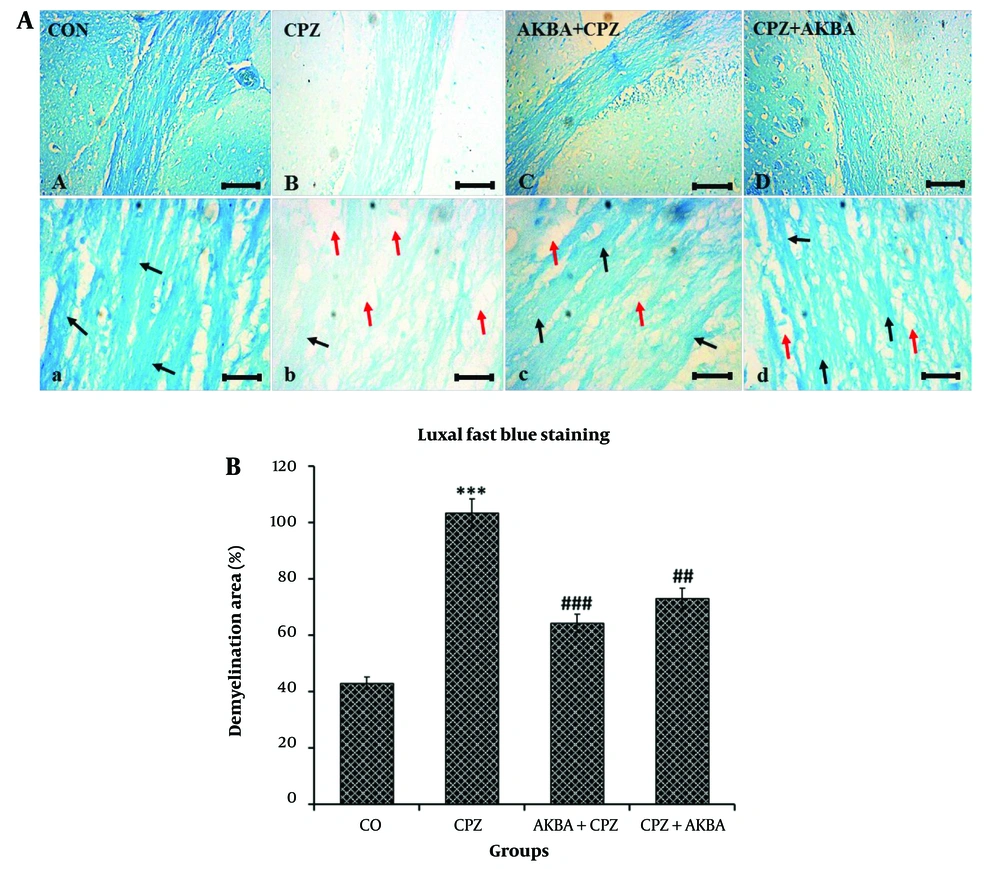
Demyelination area (DA) data are shown in Figure 2A and B. The comparison between the groups showed that DA was significantly increased in the CPZ group compared to the control group (P < 0.001). The DA was significantly reduced in the AKBA+CPZ (P < 0.001) and CPZ + AKBA (P < 0.01) groups compared to the CPZ group. In summary, the findings showed that the administration of AKBA (both pre- and post-treatment) improved demyelination in the corpus callosum region of the animal brains in the cuprizone-induced multiple sclerosis model. Pre-treatment administration of AKBA showed a more significant effect than post-treatment.
A, Light microscope micrographs of coronal sections of the corpus callosum of C57/BL6 mice brains, illustrating the extent of myelinated and demyelinated areas using the specific Luxol Fast Blue staining method. Blue areas (black arrows) indicates regions with myelin sheath density (remyelination), while white areas (red arrows) indicate the absence of myelin (demyelination). Scale bars at A, B, C, D = 100 µm, and a, b, c, d = 20 µm. B, The effect of acetyl-11-keto-beta boswellic acid demyelination area of the corpus callosum in an animal model of cuprizone-induced demyelination. Data are presented as Mean ± SEM. *** represent P < 0.001 vs. the control group; ## and ### represent P < 0.01 and P < 0.001 vs. the CPZ. CO: Control; CPZ: Cuprizone-induced demyelination model; AKBA + CPZ: CPZ group pre-treated with acetyl-11-keto-beta boswellic acid; CPZ+AKBA: CPZ group post-treated with acetyl-11-keto-beta boswellic acid.
4.4. The Effects of Acetyl-11-keto-beta-boswellic Acid on the Number of Oligodendrocytes (Olig2+ Cells) in the Corpus Callosum
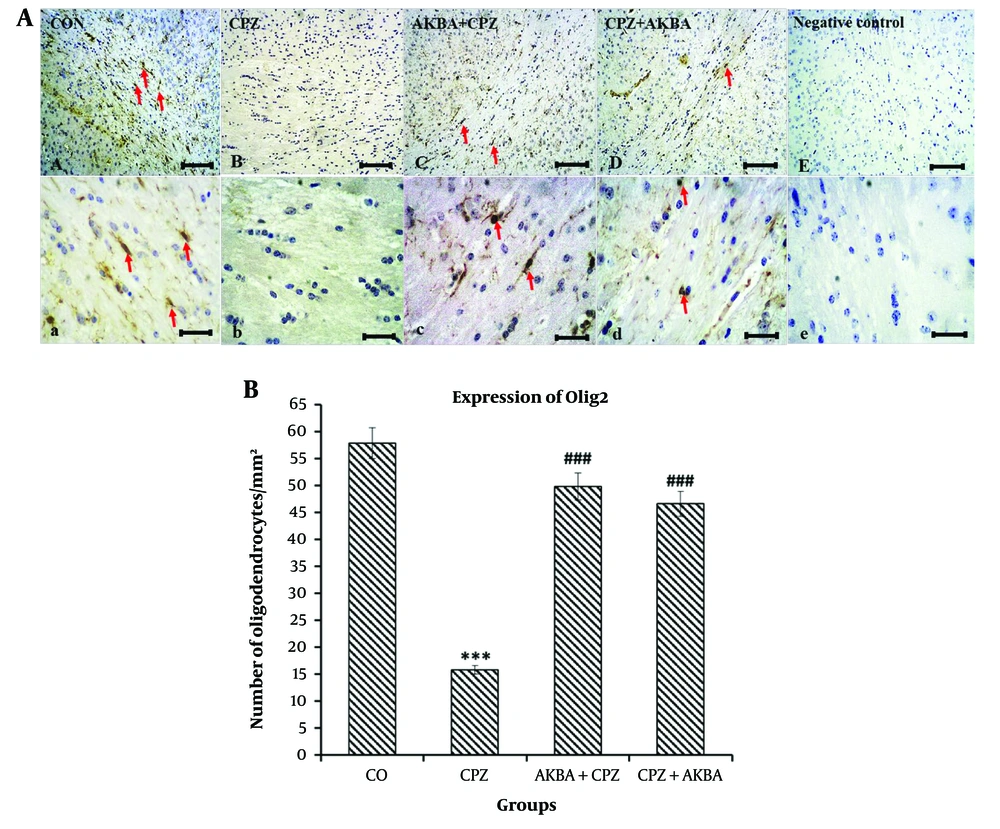
Data for the number of oligodendrocytes (Olig2+ cells) are shown in Figure 3A and B. The comparison between the groups showed that Olig2+ cells in the CPZ group significantly decreased compared to the control group (P < 0.001). The Olig2+ cells were significantly increased in the AKBA + CPZ and CPZ + AKBA groups compared to the CPZ group (P < 0.001). In summary, the findings indicate that the administration of AKBA (both pre- and post-treatment) increased the number of mature oligodendrocytes (Olig2+ cells) in the corpus callosum region of the animal brains in the cuprizone-induced multiple sclerosis model. Pre-treatment administration of AKBA showed a more significant effect than post-treatment.
A, Light microscope micrographs of coronal sections of the corpus callosum in the brains of C57/BL6 mice. The images depict oligodendrocytes (identified as olig2-positive cells) stained via immunohistochemistry for the expression of the olig2 protein. Oligodendrocyte nuclei are highlighted in brown (red arrows). Scale bars at A, B, C, D and E = 100 µm, and a, b, c, d and e = 20 µm. B, The effect of acetyl-11-keto-beta boswellic acid on the number of oligodendrocytes (per square millimeter) in the corpus callosum in an animal model of cuprizone-induced demyelination. Data are presented as Mean ± SEM. *** represent P < 0.001 vs. the control group; ### represent P < 0.001 vs. the CPZ. CO: Control; CPZ: Cuprizone-induced demyelination model; AKBA + CPZ: CPZ group pre-treated with acetyl-11-keto-beta boswellic acid; CPZ + AKBA: CPZ group post-treated with acetyl-11-keto-beta boswellic acid.
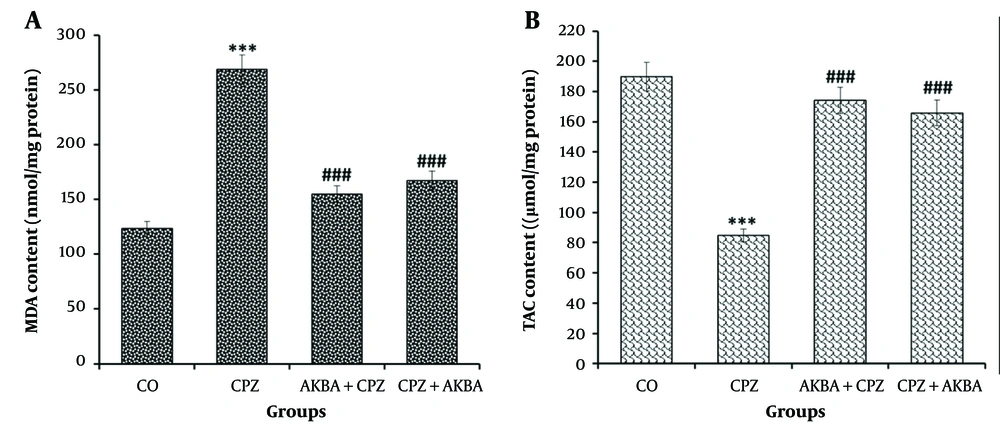
4.5. The Effects of Acetyl-11-keto-beta-boswellic Acid on the Brain Levels of Malondialdehyde and Total Antioxidant Capacity
Data on brain MDA levels are shown in Figure 4A. The comparison between the groups showed that MDA levels were significantly increased in the CPZ group compared to the control group (P < 0.001). The MDA levels were significantly decreased in the AKBA + CPZ and CPZ + AKBA groups compared to the CPZ group (P < 0.001). Data on brain TAC levels are shown in Figure 4B. One-way ANOVA on TAC showed a significant difference between the groups. Comparisons between the groups revealed that TAC was significantly decreased in the CPZ group compared to the control group (P < 0.001). Total antioxidant capacity was significantly increased in the AKBA+CPZ and CPZ+AKBA groups compared to the CPZ group (P < 0.001). In summary, the findings indicate that the administration of AKBA (both pre- and post-treatment) improved brain oxidative stress markers (MDA and TAC) in the cuprizone-induced multiple sclerosis animal model. Pre-treatment administration of AKBA showed a more significant effect than post-treatment.
The effect of acetyl-11-keto-beta boswellic acid on malondialdehyde (MDA) (A); and total antioxidant capacity (TAC) (B); levels in the corpus callosum in the animal model of cuprizone-induced demyelination. Data are presented as Mean ± SEM. *** represent P < 0.001 vs. the control group; ### represent P < 0.001 vs. the CPZ. CO: Control; CPZ: Cuprizone-induced demyelination model; AKBA + CPZ: CPZ group pre-treated with acetyl-11-keto-beta boswellic acid; CPZ + AKBA: CPZ group post-treated with acetyl-11-keto-beta boswellic acid.
5. Discussion
The important findings of this study showed that the administration of 0.3% cuprizone led to severe movement disorders, weight loss, myelin destruction, an increase in the demyelinated area in the corpus callosum, a reduced oligodendrocyte count, a decline in total antioxidant capacity, and an increase in malondialdehyde levels. Conversely, the use of acetyl-11-keto-beta-boswellic acid in the cuprizone-induced demyelination model resulted in increased resistance in the rotarod test, prevention of weight loss, a significant reduction in the amount of demyelination, diminished malondialdehyde levels, an increase in the number of oligodendrocytes, and elevated total antioxidant capacity in the corpus callosum of the brain.
Our results showed that cuprizone significantly decreased the weight and movement balance of the animals on the rotating rod in the CPZ group. In contrast, the AKBA + CPZ and CPZ + AKBA treatment groups showed a significant increase in animal weight and movement balance compared to the CPZ group, approaching the levels of the control group and indicating the effectiveness of AKBA. Notably, the AKBA + CPZ pre-treatment group demonstrated better effectiveness than the post-treatment group (CPZ + AKBA). Skripuletz et al. reported that feeding mice with cuprizone for six weeks causes weight loss, and motor coordination defects are seen in most of the mice. However, it generally does not lead to major neurological and motor defects such as muscle paralysis, with significant motor impairments occurring only where almost complete demyelination takes place (36). The weight loss and movement disorders caused by demyelination observed in the patient groups are entirely consistent with the pathological findings. Yaldizli et al. showed that damage to the corpus callosum, the largest interface between the two hemispheres of the brain, causes cognitive problems, fatigue, and motor impairments in MS, which has the highest level of demyelination (37). The results of this research suggest that the antioxidant, neuroprotective, and anti-inflammatory properties of AKBA, previously reported by Marefati in 2020, Sethi, P in 2023, Wang, Y in 2024, and Yang in 2022, may increase remyelination and nerve impulse transmission, leading to an improvement in motor function and prevention of weight loss (16, 17, 38, 39).
A general indicator of the demyelination process is the loss of the myelin sheath in the corpus callosum of the brain, characterized by specific Luxol Fast Blue staining, followed by remyelination. In this study, rats in the CPZ group experienced extensive demyelination in the corpus callosum region compared to the control group. In the control group, regular and integrated parts of nerve fibers were visible in the corpus callosum. In contrast, the CPZ group showed disorganization and disintegration of the nerve fibers, accompanied by empty spaces between them, indicating a decrease in the thickness of the myelin sheath and a subsequent reduction in the volume of the corpus callosum. Other studies have shown that the administration of 0.3% cuprizone for six weeks causes demyelination in the corpus callosum (36, 40). It should be noted that spontaneous remyelination is ineffective in MS patients, and the newly formed myelin sheath is much weaker than normal (41). In the groups receiving AKBA, the extent of the demyelinated area showed a significant decrease compared to the cuprizone group, indicating that AKBA may inhibit the myelin destruction process through its neuroprotective and anti-inflammatory properties. These findings align with the reports of Forouzanfar in 2016, Shang, P in 2016, Marefati in 2020, Sethi, P in 2023, Wang, Y in 2024, and Yang, T in 2022 (16, 21-25).
Oligodendrocytes contribute to maintaining the structural and functional integrity of axons by producing myelin sheaths in the CNS. In this study, the CPZ group showed a significant decrease in oligodendrocytes compared to the control group. The mechanism of oligodendrocyte damage in the cuprizone-induced MS model remains unknown, as this method lacks inflammatory conditions and the presence of T and B lymphocytes. However, mitochondrial dysfunction in oligodendrocytes, leading to impaired energy metabolism, is believed to cause their apoptosis (39). This rate of cell death and reduction in the number of oligodendrocytes in the cuprizone induction method can confirm various studies, including Patel and Balabanov in 2012 2. On the other hand, initial demyelination and apoptosis of oligodendrocytes likely occur in the absence of inflammation caused by T and B lymphocytes and before damage to the blood-brain barrier (3).
The number of oligodendrocytes in the AKBA + CPZ and CPZ + AKBA groups significantly increased compared to the CPZ group. Previous studies have indicated that in MS, despite the presence of oligodendrocyte progenitor cells (OPCs) in lesion areas, their insufficient differentiation into mature cells, coupled with high levels of oxidative stress and inflammatory factors, hinders effective remyelination (42). In this study, the higher number of oligodendrocytes in the AKBA+CPZ and CPZ+AKBA groups suggests that the anti-inflammatory, antioxidant, and neuroprotective properties of Boswellia extract and its derivatives, such as AKBA, likely contributed to a relative improvement in the function and differentiation of OPCs (11, 21-25, 39).
The pathological role of free radicals and oxidative stress in MS has been substantiated by various researchers (22, 25, 43). Malondialdehyde is an important marker of lipid peroxidation, and its increased level indicates a disturbance in the enzymatic and non-enzymatic antioxidant defense mechanism. In our study, administration of the neurotoxin cuprizone to mice, which causes mitochondrial defects, resulted in increased MDA levels and decreased TAC levels in the CPZ group. However, in the AKBA+CPZ and CPZ+AKBA groups, the MDA level was significantly reduced, and the TAC level significantly increased. This can be attributed to the antioxidant and neuroprotective effects of Boswellia extract and its derivatives, such as AKBA, aligning with the findings of Al-Yasiry and Kiczorowska (2016) and Hosseini-sharifabad and Esfandiari (2016) (11, 13, 17).
In general, as one of the new pieces of evidence of AKBA's mechanism of action in the brain, previous studies have shown that Boswellia Serrata extract and its constituents can reduce brain damage and protect cortical neurons against stroke in mice. This protective property was associated with a decrease in DNA oxidative damage, free radical concentration, lipid peroxidation, and glutamate concentration, along with increased glutathione levels and superoxide dismutase activity in the cerebral cortex. They also prevent the death of neurons in the CA3 region of the hippocampus in rats. It was proposed that suppression of glutamate release occurred via the inhibition of N- and P/Q-type Ca2+ channels from hippocampal synaptosomes and the suppression of protein kinase A activity (42).
Previous findings have shown that the use of AKBA increases IL-10, BDNF, superoxide dismutase, and catalase, while decreasing TNF-α, IL-6, nitric oxide, glial fibrillary acidic protein, malondialdehyde, glutamate, and peptide beta-amyloid in the brain tissue of mice, including the hippocampus, thereby restoring and strengthening memory (22). Additionally, AKBA ameliorates cognitive impairment by enhancing the Nrf2/Ho-1 signaling pathway and intercepting NF-κB-related inflammatory pathways in APPswe/PS1dE9 mice (44).
Despite the very promising results obtained in this study, its limitations include insufficient financial resources for the preparation of laboratory materials and the lack of easy access to materials such as antibodies due to their foreign nature.
5.1. Conclusions
This study represents a pioneering exploration of the effects of acetyl-11-keto-beta boswellic acid, one of the important active ingredients of Boswellia extract with strong antioxidant and anti-inflammatory properties, in the context of multiple sclerosis through comprehensive histopathological and immunohistochemical analyses. AKBA showed significant improvement in animal weight, rotarod balance, myelination rate, oligodendrocyte count, and total brain Antioxidant Index (TAC) while decreasing the Oxidative Index (MDA) in a cuprizone-induced MS mouse model. The results of this research align with previous studies on the neuroprotective, anti-inflammatory, and antioxidant effects of acetyl-11-keto-beta boswellic acid. However, the mechanism of action of Boswellia's active substances in this process requires more extensive research.