1. Introduction
Gastrointestinal (GI) cancers, including esophageal, gastric, liver, pancreatic, colon, and rectal malignancies, represent a significant global health challenge, accounting for 26.3% of all cancer diagnoses (incidence) and 35.4% of global cancer-related deaths (mortality) in 2018 (1). In 2020, Asia recorded the highest incidence rates of GI cancers, with countries like China experiencing nearly 1.5 million deaths, particularly from liver, esophageal, and stomach cancers (2). Meanwhile, Europe exhibited a higher prevalence of colorectal and pancreatic cancers, with approximately 500,000 deaths attributed to GI cancers during that year (1, 3). These regional differences may be linked to environmental factors. For example, in Asia, particularly China, hepatitis B and C infections significantly elevate the risk of liver cancer, while Helicobacterpylori infection is a major risk factor for stomach cancer, contributing to high mortality rates from these cancers (4). In contrast, Europe sees higher rates of colorectal and pancreatic cancers, largely due to dietary and lifestyle factors, such as high-fat diets and sedentary behavior. However, advanced healthcare systems in Europe facilitate earlier diagnoses and better treatment outcomes, leading to lower mortality rates despite the higher incidence of these cancers (5).
The occurrence of GI cancers has generally increased, with colon and rectal cancers now surpassing gastric cancer in terms of global impact (6). The gut microbiome plays a crucial role in the development and treatment of GI cancers, influencing both the onset of these malignancies and the efficacy of therapeutic interventions. An imbalance in microbial populations, known as dysbiosis, has been associated with an increased cancer risk, while certain microbial species may either promote or inhibit tumor development (7). The gut microbiome also plays a pivotal role in shaping responses to cancer treatments, particularly immunotherapy. Certain gut bacteria have been shown to enhance the efficacy of immune checkpoint inhibitors (ICIs) by modulating immune responses (8). Strategies such as probiotics and fecal microbiota transplantation are currently being explored to improve treatment outcomes in cancer patients (9). Probiotics, particularly Bifidobacterium, have demonstrated promising immune-modulating effects in the context of GI cancers, contributing to both prevention and therapy (9).
Bifidobacterium plays a key role in modulating the gut microbiome, which can either promote or hinder cancer progression. Alterations in the composition of gut microbiota, particularly changes in Bifidobacterium levels, have been associated with various GI malignancies, including colorectal and gastric cancers, though its overall impact remains debated (10). Some studies suggest that higher levels of Bifidobacterium may reduce cancer risk by modulating the gut environment and enhancing immune responses (11). This review aims to investigate the role of Bifidobacterium species in immune system modulation and its potential implications for protecting against GI cancers. It will focus on the mechanisms through which these probiotics may enhance immune responses and offer protection against cancer.
2. Bifidobacterium Overview
Bifidobacterium spp. are anaerobic, gram-positive bacteria that play a crucial role in maintaining gut health. These bacteria, characterized by their bifid morphology, are vital components of the human microbiota, contributing to immune regulation and various metabolic processes (12). Bifidobacterium has the potential to influence the diversity of the intestinal microbiota by modulating the abundance of microbial populations. This modulation has inhibitory effects on the proliferation of pathogenic microorganisms within the gut while fostering the growth of beneficial bacteria that promote intestinal health. Such changes are indicative of the physiological benefits associated with probiotic formulations.
Previous research has highlighted the importance of microbial communities in facilitating the recruitment of T-cells in organs, as observed in mouse models (13). Bifidobacterium plays a significant role in inhibiting tumor growth through three primary mechanisms: Stimulating the immune response, modulating the metabolic activity of intestinal probiotics to reduce carcinogen production, and promoting apoptosis in cancerous cells, thereby slowing tumor progression (14). While the precise mechanism through which Bifidobacterium and other commensal bacteria promote anti-tumor immune responses remains unclear, it is known that Bifidobacterium can enhance the effectiveness of immunotherapy. As a commensal organism, it stimulates and modulates specific pathways that influence both the innate and adaptive immune responses of the host (15).
Research indicates that Bifidobacteriumpseudolongum significantly enhances the efficacy of immune checkpoint blockade, though in the absence of ICIs, B.pseudolongum does not independently elicit an anti-tumor immune response. Notably, CD4+ T-cells in the small intestinal lamina propria showed upregulation of the Th1 master transcription factor T-bet in response to B.pseudolongum, suggesting that this bacterium has immunomodulatory properties even without the presence of ICIs (16).
Further investigations into the role of B. pseudobifidum in facilitating Th1 transcriptional differentiation during homeostasis, and its mechanisms in triggering Th1 effector functions following ICI treatment, have shown that B. pseudobifidum enhances the efficacy of ICIs through inosine. This process specifically relies on the signaling of adenosine 2A receptors (A2AR) in T-cells. Inosine, a bacterial metabolite, influences T-cells by requiring synergistic activation of dendritic cells (potentially via dendritic cells) and interleukin-12 (IL-12) receptors, which are essential for Th1 differentiation and the production of IFN-γ, thereby contributing to effective anti-tumor immunity (17).
Bifidobacteria play a vital role in the synthesis of metabolites, such as short-chain fatty acids (SCFAs), including acetate, which are utilized by various gut microbiota members. While Bifidobacteria do not directly produce butyrate, the acetate they generate modulates the activity and composition of other gut microbiota members responsible for butyrate production. Butyrate, in turn, has been shown to induce apoptosis in colorectal cancer cells, regulate the expression of anti-inflammatory cytokines such as TGFβ and IL-10 in antigen-presenting cells (APCs) and intestinal epithelial cells (IECs), and promote the development of regulatory T-cells (Tregs), contributing to a secondary butyrogenic effect (15). Further research is needed to elucidate the specific pathways that connect these mechanisms and optimize therapeutic applications.
3. Bifidobacterium and Immune Modulation
Bifidobacterium plays a crucial role in regulating immune responses through various mechanisms that promote immune tolerance and maintain homeostasis. It enhances the function of Tregs, which are essential for sustaining immune tolerance and preventing autoimmune reactions. Additionally, Bifidobacterium influences the activities of dendritic cells and macrophages, resulting in a well-regulated immune response and reduced inflammation (18).
Research into the immunomodulatory effects of Bifidobacteriumlongum KACC 91563 revealed that this strain can modulate T and B-cell proliferation and alter the balance of Th1/Th2 cytokines, including Th2 cytokines like IL-10 and IL-4, as well as Th1 cytokines such as TNF-α and IL-2. Notably, the administration of Bifidobacteriumlongum KACC 91563 led to increased levels of IgE, suggesting its role in regulating the host immune response by producing IgE and maintaining a balance between Th1 and Th2 cytokines (17).
Bifidobacteriumbifidum has been shown to induce the production of IL-12, which enhances the recruitment of tumor-specific cytotoxic T lymphocytes (CTLs) and the associated release of interferon-gamma (IFNγ) (19). Furthermore, Bifidobacteriumlactis Bl-04 has demonstrated the ability to activate antiviral immune responses in macrophages and dendritic cells, leading to increased cytokine production during viral infections. This strain has also been shown to reduce viral replication in fibroblasts, indicating potential involvement in respiratory infections (20).
Bifidobacteriumanimalis subsp. lactis improves systemic immune responses in neonatal models by modulating the gut microbiota and promoting T lymphocyte equilibrium, which is critical for effective infection control (21). Similarly, Bifidobacteriumlongum subsp. longum BB536 stimulates plasmacytoid dendritic cells, resulting in the upregulation of CD86 and HLA-DR expression and increased cytokine secretion, thereby enhancing both innate and adaptive immune responses (22).
Bifidobacterium breve CCFM1310 plays a significant role in enhancing immune responses while also influencing the composition of the gut microbiota, which is vital for maintaining overall immune health (23). The influence of Bifidobacterium on both the innate and adaptive immune systems is complex, involving various mechanisms that strengthen immune responses and support homeostasis. Different strains of Bifidobacterium regulate immune pathways, contributing to overall health (24). For example, Bifidobacteriumbifidum 791 stimulates the function of natural killer (NK) cells and T lymphocytes by upregulating activation markers like CD69 and CD25 (25). Specific responses are noted with strains like Bifidobacteriumpseudolongum, which affect lymphocyte distribution and cytokine responses, suggesting a tailored approach to immune modulation (26).
Bifidobacterium plays a critical role in regulating gut inflammation by modulating tight junctions, immune signaling, and transcriptional responses, thereby maintaining gut homeostasis (27). It strengthens the intestinal epithelial tight junction (TJ) barrier, which is essential for reducing inflammation. It modulates the expression of TJ proteins and the associated signaling pathways that maintain barrier integrity by normalizing the levels of key TJ proteins, particularly claudin-4. Additionally, it helps restore the Th1/Th2 ratio within the colonic goblet cell population, providing protection against inflammatory bowel disease (IBD) (27).
Moreover, the production of SCFAs, particularly acetate and formate, contributes to reestablishing the TJ barrier (28).
Research has demonstrated that the Bifidobacteriumlongum subsp. K5 strain has the ability to reduce inflammatory responses and protect against intestinal barrier damage caused by lipopolysaccharides (LPS). This protective effect occurs through the upregulation of mRNA expression of TJ proteins, specifically ZO-1, occludin, and claudin-1, which are vital for preserving the integrity of the intestinal barrier. Additionally, the K5 strain was found to downregulate the expression of Toll-like receptor 4 (TLR4), a key receptor involved in LPS detection, and reduce the production of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6. In a related study involving Bifidobacteriumbifidum FL-228.1, it was found that this strain protects against intestinal damage induced by dextran sulfate sodium (DSS) in murine models by significantly increasing the IL-10 to IL-12 ratio, as well as enhancing the production of mucin-2 and claudin-4 in the colon (29).
Bifidobacterium species modulate inflammatory signaling pathways, particularly the JAK/STAT and NF-kB pathways, and reduce pro-inflammatory cytokines like IL-6 and IL-1β, thereby alleviating inflammation (30). Bifidobacteriumlongum subsp. infantis influences the transcriptional landscape in IECs by enhancing anti-inflammatory signaling pathways and post-translational modifications, which govern inflammatory responses (31).
Furthermore, Bifidobacterium helps maintain gut-associated lymphoid tissue (GALT) in a state of chronic activation, facilitating the production of immunoglobulin A (IgA) responses essential for intestinal homeostasis (32). It also promotes the diversification and expansion of B cells within germinal centers, crucial for a robust immune response to pathogens (33). Additionally, Bifidobacterium modulates the gut microbiota to improve the functional metabolism of Tregs, which sustain immune tolerance and prevent colitis. This modulation enhances mitochondrial health and strengthens the IL-10-mediated suppressive capabilities of Tregs, fostering a well-regulated immune response (34).
Although Bifidobacterium exhibits significant immunomodulatory properties, further research is required to fully understand its mechanisms and their implications for health and disease.
4. Bifidobacterium in Gastrointestinal Cancer Defense
Bifidobacterium has shown promising protective effects against GI cancers through various mechanisms, including immune system modulation, induction of apoptosis, and enhancing chemotherapy effectiveness (35). A study by Li et al. demonstrated that oral administration of Bifidobacteriumbreve (B. breve) lw01 significantly enhanced apoptosis in tumor cells and suppressed tumor proliferation. This effect was contingent on the recruitment of dendritic cells (DCs) and tumor-infiltrating lymphocytes within the tumor microenvironment. The increased secretion of IL-12 from DCs was critical for the antitumor activity of B.breve, though this effect could be blocked by a neutralizing antibody against IL-12 (16).
Another study revealed a significant increase in CD8+ SIY-specific 2C T-cells within the tumor-draining lymph nodes of mice treated with Bifidobacterium, along with a notable elevation in IFN-γ production. This indicated an enhanced DC immune response prior to T-cell activation, consistent with a higher proportion of MHC-II hiDCs present in the tumors of treated mice. The study also found enrichment in pathways related to cytokine-cytokine receptor interactions, T-cell activation, and monocyte proliferation, all of which play a role in co-stimulation (such as CD40, CD70, H2M2 (MHC-I), and ICAM1). Additionally, the activation of CD8+ T-cells, DC maturation (IFNGR2, RELB), antigen presentation, and cross-presentation (TAPBP, RAB27A, SLC11A1) were also observed. Chemokine-mediated immune cells were enriched in the tumor microenvironment (CXCL9, CX3CL1, CXCR4), and the type 1 IFN signaling pathway was activated (IRF1, IFNAR2, OAS2, IFI35, IFITM1) (36).
Research also suggests that heat-inactivated Bifidobacteriumbifidum induces apoptosis in gastric cancer cells via the Akt-p53 signaling pathway. This mechanism was validated in xenograft models, where B. bifidum administration significantly delayed tumor growth (37). Additionally, Bifidobacterium longum SX-1326 was found to enhance the effectiveness of irinotecan in colorectal cancer models by influencing the p53 signaling pathway and reducing GI toxicity, thereby improving patient tolerance to chemotherapy (38).
In the subsequent sections, we will further examine the efficacy of various Bifidobacterium strains in treating different forms of digestive tract cancers.
4.1. Bifidobacterium: A Defense Mechanism Against Esophageal Cancer
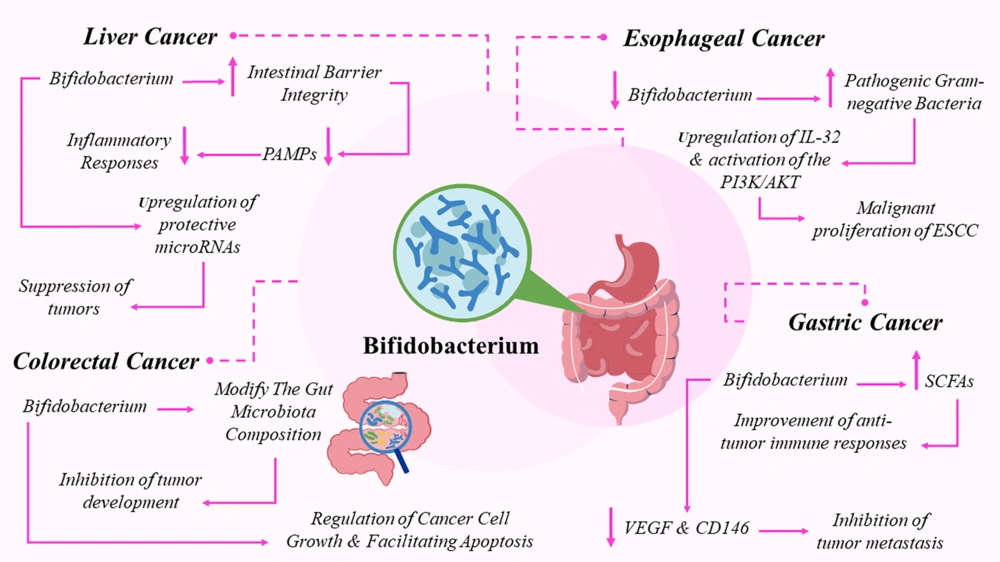
The role of Bifidobacterium in esophageal cancer (EC) is complex and influenced by its interactions with other microbial species and the host's immune responses. While specific research on Bifidobacterium in EC is limited, valuable insights can be drawn from studies on the broader esophageal microbiome (39). A decrease in microbiota diversity, especially beneficial bacteria like Bifidobacterium, has been observed in EC cases, often accompanied by an increase in pathogenic Gram-negative bacteria such as Fusobacteriumnucleatum (Fn) (39).
Fusobacterium nucleatum is known to contribute to the malignant growth of esophageal squamous cell carcinoma (ESCC) through mechanisms like upregulating IL-32 and activating the PI3K/AKT signaling pathway. This suggests that a dysbiotic microbiome, marked by a deficiency in Bifidobacterium, may facilitate cancer progression (40). Fusobacterium nucleatum infection has also been shown to suppress T-cell proliferation and cytokine secretion, weakening antitumor immune responses. The absence of beneficial microbes like Bifidobacterium may exacerbate this suppression, further reducing immune defenses against tumors (41)
In conclusion, while direct evidence of Bifidobacterium’s role in EC is limited, its potential as a protective microbe is suggested by its absence in dysbiotic conditions that promote cancer development. Further research is necessary to elucidate the specific mechanisms by which Bifidobacterium might influence EC and its potential as a therapeutic agent.
4.2. The Role of Bifidobacterium in Combating Gastric Cancer
Bifidobacterium plays a significant role in the immune defense against gastric cancer by producing SCFAs, which are essential for enhancing resistance to pathogens and regulating immune responses (42). Studies have shown that Bifidobacterium can improve the efficacy of oncolytic adenoviruses, thereby strengthening anti-tumor immune responses and reducing the infiltration of Tregs within tumors (43). Furthermore, the combination of Bifidobacteriuminfantis with herpes simplex virus-TK/ganciclovir has demonstrated an ability to inhibit tumor metastasis by lowering the levels of key proteins associated with cancer spread, such as VEGF and CD146 (44). While these mechanisms highlight the potential of Bifidobacterium in gastric cancer therapy, further research is required to fully understand its therapeutic applications and clinical effectiveness.
4.3. Liver Cancer and the Microbiome: How Bifidobacterium Contributes to Protection
Bifidobacterium plays a key role in reducing the risk and progression of hepatocellular carcinoma (HCC) through various mechanisms (45). It helps restore gut microbiota balance, addressing dysbiosis that is often linked to HCC, which can lead to liver inflammation and tumor development. By improving intestinal barrier integrity, Bifidobacterium reduces the translocation of pathogen-associated molecular patterns (PAMPs) into the liver, thereby mitigating inflammatory responses (46). Probiotics like Bifidobacterium can influence immune responses by enhancing tumor suppressor gene activity and inhibiting oncogenes. Additionally, they regulate microRNA expression, including the upregulation of protective microRNAs such as miR-122, which acts as a tumor suppressor in liver cancer by regulating apoptosis, cell metabolism, and immune responses (47). This regulation process involves precise control, particularly in pathways activated by toll-like receptors (TLRs). Studies show that microRNAs are crucial in post-transcriptional regulation of immune responses (48). Bifidobacteriumanimalis subsp. lactis Bb12, for example, has been shown to upregulate miR-15a-5p, miR-29b-3p, miR-30d-5p, and miR-181a-5p, with this effect being dependent on TLR2. Blocking TLR2 inhibits this upregulation, confirming its role in Bb12's immunomodulatory action, which starts with TLR2 recognition of bacterial membrane components like peptidoglycans or lipoteichoic acid, leading to activation of transcription factors such as NF-κB and AP-1 (48). Bifidobacterium also exerts direct antitumor effects by binding to carcinogenic substances and producing metabolites that inhibit cancer cell proliferation. Its antioxidant and anti-inflammatory properties further reduce the risk of HCC development (49). More research is needed to fully understand these mechanisms and optimize Bifidobacterium’s therapeutic applications in managing HCC.
4.4. Bifidobacterium’s Influence on Colorectal Cancer
Various mechanisms employed by Bifidobacterium species, particularly Bifidobacteriumlongum and Bifidobacteriumadolescentis, play significant roles in both the progression and treatment of colorectal cancer (CRC) (50). Bifidobacteriumlongum has the ability to modify the gut microbiota composition, which is crucial for maintaining intestinal health and reducing the risk of CRC. Research suggests that it can restore dysbiosis in CRC models, promoting beneficial microbial communities that inhibit tumor growth (38). Additionally, Bifidobacteriumlongum has been shown to enhance immune function, which may help regulate cancer cell growth and induce apoptosis. It activates the p53 signaling pathway, leading to the upregulation of pro-apoptotic factors like Cleaved Caspase-3 while downregulating anti-apoptotic factors such as Bcl-2 (38, 50).
Studies also show that both viable and heat-inactivated Bifidobacteriumlongum can increase apoptosis in colon cancer cells by inducing reactive oxygen species (ROS) and disrupting mitochondrial membrane potential (14). Moreover, Bifidobacteriumadolescentis influences cancer-associated fibroblasts, which play a key role in regulating the tumor microenvironment, thus contributing to the suppression of CRC (51). These findings highlight the potential of Bifidobacterium in managing CRC; however, the complex interactions within the gut microbiota and the variability in individual responses to probiotics require further investigation to fully understand their therapeutic value. Figure 1 provides a comprehensive overview of the impact of Bifidobacterium on GI cancers.
5. Nutritional Interventions in Gastrointestinal Cancer Prevention and Therapy
Nutritional interventions play a critical role in both the prevention and treatment of GI cancers, contributing to improved patient outcomes, reducing side effects, and potentially enhancing treatment effectiveness. Dietary approaches are increasingly recognized as integral to holistic cancer management (52). Research suggests that nutritional support can mitigate the adverse effects of concurrent chemoradiotherapy. One study demonstrated that patients receiving nutritional interventions experienced fewer incidences of radiation esophagitis and maintained better nutritional status compared to those without support (53). Additionally, modulating the gut microbiota and employing immunonutrition may improve recovery and therapeutic responses, with certain nutrients potentially working synergistically to enhance treatment efficacy (54).
Targeted dietary approaches aimed at altering gut microbiota have been shown to increase the abundance of beneficial bacteria, including Bifidobacterium, which may play a role in cancer prevention (55). Probiotics, particularly strains like Lactobacillus and Bifidobacterium, have shown promise in reducing tumor development and progression in colon cancer models. For instance, pre-administration of Lactobacillus resulted in an 86.21% decrease in tumor formation rates in murine studies (56). Combining probiotics and prebiotics enhances immune responses and supports the growth of beneficial gut bacteria, which may suppress cancer cell growth (57).
While diets rich in fiber, fruits, and low in fat have been associated with changes in gut microbiota composition, studies on their effects on bacterial diversity in rectal tissue have produced inconsistent results (58). However, diets abundant in fruits and vegetables have been linked to an increase in beneficial bacteria like Bifidobacterium, which correlates with a lower risk of cancer (59). Integrating dietary modifications and Bifidobacterium supplementation with standard GI cancer treatments offers the potential to significantly improve patient outcomes by optimizing nutritional status, modulating immune responses, and reducing treatment-related side effects (54).
6. Challenges and Future Directions
Bifidobacterium, a well-known probiotic, plays a critical role in regulating the immune system, particularly in the context of GI malignancies. Studies suggest that Bifidobacterium may enhance the effectiveness of cancer immunotherapy and impact tumor development through multiple pathways (35). Preclinical evidence supports the potential of Bifidobacterium in cancer protection, especially in GI cancers, and suggests that incorporating dietary changes and Bifidobacterium supplementation alongside conventional therapies could improve patient outcomes by boosting nutritional health, regulating immune responses, and mitigating the side effects of treatment. However, several challenges remain in translating these findings to human trials and clinical practice, including the complexity of host-microbe interactions, inter-individual variability, regulatory hurdles, and clinical integration.
The intricate relationship between Bifidobacterium and the host's immune system is a significant challenge. Understanding the precise mechanisms through which Bifidobacterium modulates immune responses requires extensive research, considering the diverse characteristics of the gut microbiome. Different strains of Bifidobacterium may exhibit varying levels of effectiveness in influencing immune responses and addressing GI cancers, making it essential to identify the most potent strains and understand their specific functions.
The effectiveness of Bifidobacterium in preventing and treating cancer may also vary between individuals, influenced by genetic predispositions, environmental factors, and lifestyle choices. Each person has a unique gut microbiome, which can significantly impact the colonization and effectiveness of administered Bifidobacterium strains. Pre-existing microbial communities may either compete with or support the growth of introduced Bifidobacterium, affecting its ability to modulate the immune system and exert anti-cancer effects. Additionally, the diversity and stability of an individual's gut microbiome can influence how well they respond to probiotic interventions (60).
Genetic variations in immune-related genes can further affect how Bifidobacterium modulates the immune response against cancer cells. Polymorphisms in genes involved in bacterial recognition and processing (e.g., pattern recognition receptors) may influence host-microbe interactions and the subsequent immune activation (61).
The general health of a patient, including their nutritional status, significantly impacts the effectiveness of Bifidobacterium-based therapies. Comorbidities, such as IBDs or metabolic disorders, can alter the gut environment, affecting the colonization and function of administered Bifidobacterium. Additionally, the stage and type of cancer may influence a patient's response to probiotic interventions (27). Factors such as prebiotic intake, fiber consumption, and overall dietary patterns may either support or hinder the growth and activity of administered Bifidobacterium strains. Lifestyle aspects like stress levels, sleep patterns, and physical activity also play a role in shaping the gut microbiome and immune function (58).
Previous or ongoing cancer treatments, including chemotherapy or radiation therapy, can modify the gut microbiome and potentially affect the colonization and efficacy of Bifidobacterium. Moreover, the concurrent use of antibiotics or other medications may interfere with the establishment of probiotic strains (38). Age-related changes in gut microbiota and immune function, along with sex-specific differences in immune responses and microbiome composition, may also contribute to variations in treatment outcomes (62).
Given these factors, it is essential to adopt personalized strategies that tailor probiotic interventions to the specific needs of each individual. The use of Bifidobacterium as a therapeutic agent requires careful regulation to ensure both safety and efficacy.
Comprehensive long-term studies are essential to evaluate potential adverse effects and to develop standardized protocols for clinical implementation. Integrating Bifidobacterium into current cancer treatment regimens presents challenges related to optimizing therapeutic protocols and understanding potential interactions. Collaborative efforts between microbiologists, oncologists, and immunologists are crucial to addressing these issues effectively. Future research should focus on elucidating the molecular pathways through which Bifidobacterium influences immune responses and inhibits cancer progression. Advanced techniques such as metagenomics and metabolomics may provide valuable insights.
Despite extensive research in animal models, there is a significant lack of human studies. Comprehensive clinical trials are needed to assess the effectiveness of Bifidobacterium-based therapies in preventing and managing GI cancers. These studies should consider factors such as strain specificity, dosage, and treatment duration. Developing personalized probiotic treatments that account for individual microbiome profiles and genetic factors may enhance the efficacy of Bifidobacterium in cancer prevention and treatment and should be a key focus of future research.
Investigating synergistic strategies that combine Bifidobacterium with other probiotics, prebiotics, or conventional therapies could improve treatment outcomes. Future research should aim to identify the most effective combinations and treatment protocols. Public health initiatives that promote gut health through dietary choices, lifestyle modifications, and probiotic supplementation may also play a significant role in cancer prevention. Educational campaigns can raise awareness about the benefits of Bifidobacterium.
Many current studies have limited follow-up periods. Long-term investigations that monitor changes in the gut microbiome and immune system over extended periods can provide critical insights into the role of Bifidobacterium in cancer prevention and progression. Additionally, there is a notable gap in research on the effects of various Bifidobacterium strains on specific GI cancers, particularly pancreatic cancer. Addressing these gaps and exploring future research directions will enable Bifidobacterium to reach its full potential as a key player in the fight against GI cancers, leading to the development of novel and effective therapeutic strategies.
7. Conclusions
In summary, this review highlights the crucial role of Bifidobacterium in combating GI cancers, demonstrating its diverse mechanisms in enhancing immune responses, regulating gut microbiota, and potentially improving the efficacy of cancer treatments. The key findings emphasize that Bifidobacterium not only supports gut health but also offers protective effects against various malignancies through immune modulation, induction of apoptosis, and enhancement of treatment effectiveness. However, the complex nature of its interactions requires further research to explore the therapeutic potential of different Bifidobacterium strains, with the aim of optimizing their application in cancer prevention and treatment strategies.