1. Background
Pleural effusion, empyema, and pneumothorax are adverse outcomes of certain clinical conditions affecting the lungs and chest, often associated with high mortality rates. Pleural effusion refers to the accumulation of fluid in the pleural space (1, 2), and malignant pleural effusion (MPE) is particularly severe, with a mortality rate of up to 75% (3).
Empyema is the accumulation of purulent fluid in the pleural space, often caused by pneumonia, and can also occur after surgery or chest trauma (4). It is associated with increased complications and mortality, with approximately 20% to 30% of patients either succumbing to the condition or requiring re-surgery within the first year after empyema complications. Therefore, early intervention is crucial (5).
Pneumothorax refers to the accumulation of air in the pleural cavity outside the lung, which can build up and exert pressure on the lung, causing partial or complete collapse. Pneumothorax can be traumatic or non-traumatic. Non-traumatic spontaneous pneumothorax is further categorized as primary or secondary (6).
Primary spontaneous pneumothorax (PSP) is usually benign and resolves without intervention. However, the recurrence rate is about 30% for PSP and 43% for secondary spontaneous pneumothorax. Primary spontaneous pneumothorax is not considered a significant health threat and rarely results in mortality. In contrast, secondary pneumothorax carries a higher mortality risk of up to 10%, depending on the underlying conditions and the extent of the pneumothorax (6).
Management of these complications typically involves surgical interventions. In empyema, as in all infections, rapid initiation of antibiotics and control of the infection source are essential. Thoracostomy and drainage are the most common methods employed. Larger lumen tubes show no difference in mortality and prognosis compared to smaller tubes but are associated with more pain. Tubes smaller than 14F are more applicable and often achieve recovery within the first 24 hours. If excessive discharge occurs, more invasive methods using larger tubes or surgery become inevitable (7).
In recent years, the use of wire-guided chest drains (Seldinger technique) has gained popularity (8). The British Thoracic Society recommends small-lumen catheters (10 to 14F) for the treatment of pneumothorax (9) and malignant pleural effusion (10). However, there is limited information from controlled studies on the effectiveness of these drains, and no consensus exists regarding their use in empyema treatment (11, 12).
Although placing wire-guided chest drains through a very small incision is less harmful to patients and less invasive than larger lumen drains, their effectiveness in pleural effusion and empyema has not been definitively confirmed in numerous studies (9, 11). Additionally, only a few studies with large sample sizes have been conducted in this area (13, 14).
Drainage using small-bore wire-guided chest drains has been considered a suitable treatment for patients with pleural fluid accumulation, empyema, or pneumothorax.
2. Objectives
The novelty of this study lies in comparing this method with traditional approaches. The technique using small-bore wire-guided chest drains has proven to be a safe and tolerable method for managing pneumothorax. If successful for empyema and pleural effusion, it could serve as an effective alternative to traditional chest tubes due to its less aggressive nature and shorter recovery period.
3. Methods
In a quasi-experimental study with a descriptive analytical approach conducted at Kowsar Hospital, affiliated with Semnan University of Medical Sciences, patients hospitalized with a definitive diagnosis of pneumothorax, empyema, or malignant pleural effusion were enrolled using a convenience sampling method. Patients with evidence of chest trauma (hemothorax), acute empyema, sepsis, or an unstable condition were excluded from the study.
The medical records of patients with confirmed diagnoses of pneumothorax, empyema, or malignant pleural effusion were reviewed. Kowsar Hospital serves as a referral center for heart and chest surgery in the area covered by Semnan University of Medical Sciences. The study sample included 101 patients with pneumothorax, empyema, or malignant pleural effusion who were referred with clinical and radiological evidence of these conditions between April 2022 and the end of September 2023 for treatment (palliative or symptomatic) via thoracentesis and pleural fluid drainage using a small-bore wire-guided chest drain (double or triple lumen central venous catheter set, manufactured by ARROW, USA).
Patients who underwent radiography followed by ultrasound of the pleural effusion before catheter placement were examined. Those who met the inclusion criteria, which included positive ultrasound findings indicative of pleural effusion, were included in the study. Patients referred for thoracic surgery or with evidence of chest trauma (hemothorax) were excluded.
The basic demographic and clinical data, including age, gender, underlying diseases, blood pressure, and hyperlipidemia, were recorded in a checklist.
In cases of empyema, the diagnosis was confirmed through pleural fluid analysis and pathological findings. For malignant pleural effusion (ME) cases, the main inclusion criterion was that the technique was used as a first-step therapeutic method.
The thoracentesis procedure involved placing patients in a supine position. Local anesthesia was administered using 100 to 200 mg of 2% lidocaine solution, and the drain was inserted either from the medial line of the clavicle in the second or third intercostal space or in the fourth, fifth, or sixth intercostal space along the mid-lateral line. The drain was secured to the skin using 1-0 or 0-0 silk sutures.
Drainage was established by attaching the drain to a specialized bottle, with aspiration applied if needed in cases of pneumothorax. Prophylactic antibiotics were administered to all patients immediately before the procedure. After the drain was placed, chest radiography (CXR) was performed to confirm the correct position of the drain within the pleural cavity. Clinical evaluations were conducted three days post-procedure using a CXR.
Data on minor and major complications, catheter removal time, hospital stay duration, and treatment outcomes (including the need for complementary treatments due to treatment failure) were recorded.
Major complications of chest tube insertion included lung penetration, significant cardiovascular perforation, diaphragm perforation, intercostal vascular perforation, chest empyema (in cases of pleural effusion or pneumothorax), and other complications leading to prolonged hospitalization. Minor complications included incision site infection, hypotension, and hemorrhage.
Treatment failure was defined by one of the following conditions:
(1) incomplete placement or displacement of the drain requiring removal and replacement,
(2) pneumothorax (if it was not the initial reason for referral and drain placement), and
(3) drain blockage or leakage.
The drain was removed when daily fluid drainage decreased to less than 100 mL for ME cases or when no air leakage was observed, with full lung expansion and no aspiration required (typically after three days) in cases of pneumothorax.
3.1. Statistical Analysis
Descriptive statistics (frequency and percentage) were used to describe categorical variables. Quantitative variables were presented as the mean, standard deviation, maximum, and minimum values. A chi-square test was used to compare categorical variables between groups. Comparisons of quantitative variables between groups were conducted using the independent t-test or its nonparametric equivalent, the Mann–Whitney U test.
The chi-square test was also employed to estimate the incidence of complications in gender and age subgroups. For all statistical tests, a significance level of less than 0.05 was considered. Data analysis was performed using SPSS statistical software, version 24.
4. Results
The study included 101 patients referred to Kowsar Hospital during 2022–2023 who underwent thoracentesis using small-bore wire-guided chest drains for the treatment of pneumothorax, malignant pleural effusion, and empyema.
The mean age of the patients was 73.08 ± 15.63 years (range: 21 - 99 years). Based on the redefinition of the elderly as individuals older than 75 years (15), the patients were divided into two groups: Those aged 75 years and younger (51 patients, 50.5%) and those older than 75 years (50 patients, 49.5%). Of the total, 58 patients (57.4%) were male, and the remaining were female. The most common indication for catheter placement was pleural effusion. In five patients, catheter placement was due to malignant pleural effusion, and in one patient, it was required for empyema.
The most common underlying disease among patients was hypertension, observed in 43 cases (42.6%). Ischemic heart disease was the second most common condition, affecting 37 patients, followed by diabetes in 35 patients. The demographic characteristics of the patients are summarized in Table 1.
| Characteristics | No. (%) |
|---|---|
| Gender | |
| Male | 58 (57.4) |
| Female | 43 (42.6) |
| Age (y) | |
| 75 ≥ | 51 (50.5) |
| > 75 | 50 (49.5) |
Distribution of Patient Abundance Based on Demographic Profile
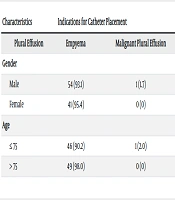
The most common indication for catheter placement was pleural effusion. In five patients, catheter placement was due to malignant pleural effusion, and in one patient, it was needed for empyema. Among male patients, pleural effusion was the indication for catheter placement in 54 cases (93.1%), while one case had empyema, and three cases had malignant pleural effusion. Among female patients, pleural effusion was the cause of catheter placement in 41 cases (95.4%), and two cases were due to malignant pleural effusion. The indications for catheter placement did not differ significantly between men and women (P = 0.681) (Table 2).
| Characteristics | Indications for Catheter Placement | P-Value | ||
|---|---|---|---|---|
| Plural Effusion | Empyema | Malignant Plural Effusion | ||
| Gender | 0.681 | |||
| Male | 54 (93.1) | 1 (1.7) | 3 (5.2) | |
| Female | 41 (95.4) | 0 (0) | 2 (4.6) | |
| Age | 0.236 | |||
| ≤ 75 | 46 (90.2) | 1 (2.0) | 4 (7.8) | |
| > 75 | 49 (98.0) | 0 (0) | 1 (2.0) | |
Comparison of the Frequency of Indications for Catheter Placement in Patients Based on age and Gender a
In the age group of 75 years and younger, the indication for catheter placement was pleural effusion in 46 patients (90.2%), malignant pleural effusion in 4 cases (7.8%), and empyema in 1 case (2%). In patients older than 75 years, the most common indication for catheter placement was pleural effusion, observed in 49 cases (98%), while one case (2%) required catheter placement due to malignant pleural effusion. The frequency of different indications for catheter placement did not differ significantly between the two age groups (P = 0.236).
The mean duration from catheter placement to removal was 7.84 ± 10.7 days (range: 2 - 40 days). Among the patients who underwent catheter placement, 16 (15.8%) died during the follow-up period, and 5 (4.9%) experienced complications related to catheter placement (Table 3). These complications included catheter obstruction in 2 patients, pneumothorax in 2 patients, and hemothorax in 1 patient.
| Outcomes | No. (%) |
|---|---|
| Mortality | 16 (15.8) |
| Complications | |
| Total | 5 (5.0) |
| Obstruction | 2 (2.0) |
| Pneumothorax | 2 (2.0) |
| Hemothorax | 1 (1.0) |
Frequency of Mortality and Complications After Catheter Placement
The case of hemothorax occurred in a 79-year-old woman who underwent catheter placement for pleural effusion associated with underlying pneumonia. Of the two cases of pneumothorax, the first involved a 91-year-old man with underlying hypertension who underwent catheter placement for pleural effusion six days prior. He was treated with a chest tube for pneumothorax but unfortunately died the following day. The second case was a 21-year-old man without underlying conditions who developed pneumothorax after catheter placement for empyema.
Catheter blockage occurred in 2 patients. The first was a 75-year-old woman with multiple underlying conditions, including hypertension, diabetes mellitus, hyperlipidemia, and cerebrovascular accident, who underwent catheter placement for pleural effusion. The second case was a 77-year-old man with bowel cancer, hyperlipidemia, hypertension, and pulmonary involvement. He underwent catheter placement for pleural effusion but required a chest tube due to incomplete drainage.
5. Discussion
In a study by Abuejheisheh et al., the mean catheter duration was 4.14 ± 2.85 days (16). In the study by Congedo et al., the thoracic drain was removed after a mean period of 7.20 ± 8.87 days, either following the resolution of pleural effusion or due to tube displacement (17).
Chest catheters and drains are typically used to remove fluid from the pleural cavity. Chest catheters can be removed when there is no empyema or air leakage, and the drainage volume has decreased to an acceptable level. Patients are rarely discharged from the hospital with a chest tube in place; thus, earlier removal can result in a shorter hospital stay (18).
In our study, the mean age of the patients was 73.08 ± 15.63 years, with half of the patients being older than 75 years. Of the participants, 58 (57.4%) were male, and the rest were female. In the study by Cafarotti et al., patients who had undergone placement of a small-bore wire-guided chest drain had a mean age of 55.85 ± 18.6 years, and 61.7% were male (19). In the study by Horsley et al., the mean age was 64 ± 2 years (14). Similarly, in the study by Abuejheisheh et al., 75.3% of patients were male, the mean age was 56.85 ± 13.18 years, and the most common indication for chest drain placement was cardiac surgery (84.8%, 134 cases), followed by pleural effusion (6.3%, 10 cases) (16).
In our study, the most common indication for catheter placement was pleural effusion. Similarly, in the study by Horsley et al., pleural effusion was the indication for catheter placement in 52% of cases (14). Indications for catheter placement include pneumothorax, hemothorax, pleural effusion, and empyema, with the procedures sometimes performed under the guidance of ultrasound, CT scan, fluoroscopy, or a combination of these techniques (20).
The mean age of the patients in our study was higher than in other studies, which may be attributed to the unique conditions of our center. As a referral center in Semnan Province, this facility typically treats patients with multiple complications, underlying diseases, and advanced age. In our study, the most common underlying disease in patients undergoing catheter placement was hypertension, affecting 42 patients (42.6%). This was followed by ischemic heart disease in 37 cases and diabetes in 35 cases.
Among the patients who underwent catheter placement, 16 (15.8%) died during the follow-up period, and 5 (4.9%) experienced various complications associated with catheter placement. These complications included catheter obstruction in 2 patients, pneumothorax in 2 patients, and hemothorax in 1 patient. The incidence of complications was not associated with gender (P = 0.902) or patient age (P = 0.630).
Orlando et al. reported that the prevalence of complications following small- and large-bore catheter placement was 14% and 18%, respectively. Additionally, the need for video-assisted thoracic surgery was higher in patients with large-bore catheters, while pneumonia was more common in those with small-bore catheters (21). Similarly, Congedo et al. found that 6.5% of patients had complications following the placement of a SBWGD, including one case of pneumothorax and three cases of displacement and obstruction (17). In the study by Corcoran et al., the use of SBWGD was associated with few adverse outcomes and was described as a safe and efficient method (22).
In the study by Davies et al., minor serious complications following SBWGD insertion were displacement and obstruction (13). Previous studies have reported the rate of displacement and blockage to range between 0.2% and 6% (13).
Our study similarly found that small-bore catheter placement is associated with few complications. The Seldinger technique is preferred for chest drainage, and SBWGD are generally considered effective and safe.
In some studies, serious complications and deaths have been reported following partial thoracic drainage and catheter placement. One such study documented 12 deaths and 15 serious complications over a three-year period from 2005 to 2008 (23). Harris et al. reported mortality in seven patients following chest catheter placement for various indications, with the main causes being improper placement and severe lung or chest wall damage (24).
In the study by Kamio et al., which assessed complications of thoracentesis and chest tube placement over a ten-year period, 15 patients (11%) died, with all cases resulting from severe complications (25). Similarly, the Treml et al. study observed complications in patients for whom a small-bore chest drain (SBCD) was used for pleural effusion. The most common complication was pneumothorax (4.5%), followed by bleeding (0.8%). Women and lighter-weight patients were found to have a higher risk of complications. The mortality rate in this study was 22%, with higher rates among patients admitted to intensive care units and those in the uncomplicated group (26).
In our study, mortality during the follow-up period after chest catheter placement was relatively high but was not related to the incidence, type of complications, or gender of the patients. Notably, these deaths were unrelated to catheter placement complications. Most mortality cases occurred in patients over 75 years of age who also had underlying conditions, often multiple, which could have contributed to their deaths.
Treml et al. similarly reported high mortality rates but noted that none of the deaths were directly attributed to pleural effusion drainage procedures. Instead, the mortality cases were mainly among patients who were critically lll (based on the Simplified Acute Physiology Score, SAPS II), had been admitted to intensive care units, or had a history of ICU admission. These deaths were not directly associated with complications from pleural effusion drainage (26).
One contributing factor to the increased mortality rates in these patients is the disruption of oxygen delivery. Prospective studies with smaller sample sizes have investigated the association between oxygenation disruption and clinical outcomes. Mattison et al. evaluated 100 ICU patients with pleural effusion and found that they had longer ICU stays and extended periods of mechanical ventilation (27). Similarly, Bateman et al. reported that malignant pleural effusion is associated with increased mortality, and pleural drainage procedures can sometimes exacerbate this mortality (28). However, drainage is often essential for diagnosis and appropriate treatment, which can ultimately improve outcomes (29).
In our study, none of the patients died as a direct result of the pleural drainage technique. The mortality rate of 15.7% was primarily observed in elderly patients with numerous underlying diseases. Uncomplicated patients also exhibited higher mortality rates. Given the low percentage of mortality in uncomplicated patients, caution should be exercised when interpreting and promoting these findings. The mortality rates observed in our study are slightly better than those reported in a European multi-center cohort study, where overall mortality was reported at 19.5% (30). Fysh et al. demonstrated that early drainage had no significant effect on mortality, length of stay in the intensive care unit, or overall duration of hospitalization (31).
Typically, large-bore chest tubes (LBCT) are used in situations where there is a risk of drain blockage, such as empyema or active bleeding, and in cases of chest trauma leading to hemothorax (32). Additionally, LBCTs are employed in cases of traumatic pneumothorax when the patient is under mechanical ventilation (33). In these instances, the incidence of complications and mortality has been reported to be relatively high (24, 34, 35).
It can be inferred that high mortality in these patients is not directly related to the drainage method. Therefore, given the low complication rate observed, the use of SBWGD appears to be a safe and efficient method, particularly for deteriorated patients. While pleural effusion drainage is not a standalone treatment, it serves as an auxiliary procedure widely employed in acute respiratory care.
5.1. Study Limitations
This study has several limitations. First, its retrospective design did not allow for differentiation between emergency, non-emergency, and elective cases requiring drain placement. Second, the consequences of drain placement on a daily basis were not monitored until the drain was removed, and only the final outcomes and the occurrence of complications were recorded during the follow-up period. Third, the patients' conditions were not systematically assessed for deterioration at the time of drain placement, despite the availability of criteria to determine clinical deterioration. Most importantly, this study exclusively examined the conditions, outcomes, and consequences of one type of drain, the SBWGD, without comparison to other methods. As a result, its effectiveness cannot be confidently stated in the absence of comparative data.
5.2. Conclusions
Our study, which assessed the efficacy and outcomes of small-bore wire-guided chest drains in the treatment of malignant effusion and pleural empyema, demonstrated that this method was associated with few complications, and these complications were not related to the gender or age of the patients. While the mortality rate during the follow-up period was relatively high, most deaths occurred in elderly patients and those with multiple underlying diseases, and this cannot necessarily be attributed to the outcomes of drain placement.
The use of small-bore wire-guided chest drains in the treatment of malignant effusion and pleural empyema is a safe and low-risk method. It can be recommended in similar situations, particularly for the treatment of pleural effusion in middle-aged patients.