1. Background
The global spread of the COVID-19 virus has led to its classification as a pandemic, posing a major public health emergency (1). SARS-CoV-2, identified as Severe Acute Respiratory Syndrome Coronavirus-2, is the recently discovered coronavirus responsible for the emergence of COVID-19 (2). The worldwide pandemic has presented a significant challenge to healthcare systems across the globe (3). The illness can range from a mild acute respiratory infection to a severe case of pneumonia with respiratory failure, acute respiratory distress syndrome (ARDS), hypercoagulation, septic shock, and ultimately death (4). A full understanding of the underlying causes of the observed differences in disease manifestations and outcomes is still lacking (5). Research indicates that older adults and individuals with underlying health conditions, such as heart disease and diabetes, are more susceptible to developing severe symptoms of COVID-19 (6). In contrast, pediatric cases tend to follow a less severe clinical course, while older age and male gender have been associated with a poorer prognosis (7). The outbreak of COVID-19 has directly impacted individuals with diabetes, leading to an increase in acute diabetic complications and placing additional strain on healthcare services (8). Diabetes mellitus is a chronic metabolic disorder characterized by persistently high blood glucose levels (9). It is associated with severe long-term complications, including heart disease and chronic kidney problems (10). Several studies have demonstrated the link between type 2 diabetes, the most common form of the disease, and chronic low-grade inflammation, which affects blood sugar control and insulin sensitivity while also influencing the body’s innate and adaptive immune responses (11).
Research has shown that elevated blood sugar levels, known as hyperglycemia, can significantly impact the outcomes of COVID-19. It has also been identified as a potential risk factor for predicting the need for intensive care unit (ICU) admission and poor outcomes in affected individuals (7). According to one study, during the pandemic, 69.7% of individuals with diabetes had uncontrolled blood sugar, with their HbA1c levels rising from 7.8 to 8.1 (12). Another study found that individuals with type 2 diabetes infected with COVID-19 experienced significant disturbances in glycemic regulation, as evidenced by elevated fasting glucose levels and increased HbA1c percentages during the infection, compared to their pre-infection measurements. After recovering from COVID-19, these parameters showed a marked decrease, but remained higher than pre-infection values, suggesting a lingering effect on glycemic management (13). Additionally, disruptions in lipid metabolism may contribute to the progression of COVID-19 (14). Recent research has also indicated that lipid levels decrease in individuals with mild COVID-19 symptoms and worsen as the disease advances to more severe stages (15).
2. Objectives
The aim of this analytical cross-sectional study was to explore the relationship between the COVID-19 outbreak and metabolic parameters, specifically focusing on hemoglobin A1c (HbA1c) levels and lipid parameters, including total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglyceride levels.
3. Methods
3.1. Design and Settings
This cross-sectional study aimed to assess 64 adult diabetic patients, aged 18 years and older, who sought medical care at least once between March 2021 and March 2022 at the Endocrinology and Metabolism Clinic at the Kowsar Education, Research, and Treatment Center, affiliated with Semnan University of Medical Sciences. The sample size represents the total number of eligible diabetic patients who attended the clinic during the study period. The exclusion criteria included refusal or retraction of informed consent, pregnancy, and being under 18 years of age. Participants were selected using a total population sampling technique. This approach ensured that all eligible diabetic patients diagnosed with diabetes and referred to the clinic during the study period were enrolled. Diagnosis of diabetes was based on ADA criteria and/or information provided in their medical history, including insulin use or the use of oral hypoglycemic medications (16). The individuals included in the study were diagnosed with COVID-19 either by testing positive for the virus through a reverse-transcription polymerase chain reaction (RT-PCR) test of the upper respiratory tract or by a specialized team that identified clinical symptoms along with high-resolution computed tomography (HRCT) scan results. There were no restrictions on participation for people with diabetes, including individuals with either type 1 or type 2 diabetes.
3.2. Data Collection
A specific checklist was used to examine all the diabetic individuals participating in the study. This checklist included various aspects, such as demographic information (gender and age), the duration of diabetes diagnosis, the type of treatment (insulin or oral hypoglycemic agents), levels of hemoglobin A1c (HbA1c), lipid parameters (total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides), and whether or not the individual had contracted COVID-19. All data was collected and documented over a one-year period, from March 2021 to March 2022.
3.3. Ethical Considerations
The research was approved by the Ethics Committee of Semnan University of Medical Sciences (No. IR.SEMUMS.REC.1401.045) in Semnan, Iran. Prior to participation, all patients provided written informed consent. The data extracted from the patients' records was accessible only to the research project leader, ensuring strict confidentiality. This data was used solely for statistical analysis and for no other purposes.
3.4. Statistical Analysis
The statistical analysis was performed using IBM SPSS Statistics software (version 26) on a Windows platform and GraphPad Prism software (version 8.4.3). A range of variables were analyzed, categorized into categorical and continuous groups. Categorical variables were described using frequencies and percentages, while continuous variables were summarized using various descriptive statistics, such as medians, modes, means, standard errors of the mean, standard deviations, interquartile ranges, and variances. The normality of the data was assessed using the Kolmogorov-Smirnov normality test, which confirmed that the data followed a normal distribution. A P-value less than 0.05 was considered statistically significant. The Pearson correlation coefficient and two-tailed P-value were used to examine the associations between the variables. Differences between groups were compared using a one-way analysis of variance (ANOVA). The chi-square test was used to analyze categorical variables, and the Mann-Whitney U test was applied to compare continuous variables between the two groups.
4. Results
A total of 64 individuals met the eligibility criteria for participation in the study. However, due to incomplete descriptive data for 3 individuals, only 61 participants were included in the analysis, with the remaining 3 individuals classified as missing data. Among the 61 participants, 20 (32.8%) were male and 41 (67.2%) were female. Of the 61 patients examined, 5 (8.2%) were prescribed insulin treatment, while 56 (91.8%) were prescribed oral medication. Furthermore, 12 individuals (19.7%) tested positive for COVID-19, while 49 (80.3%) tested negative for the virus. The average age of the participants was 53.92 years (SD = 9.301), with ages ranging from 35 to 74 years. The mean HbA1c level among the patients was 7.495 (SD = 0.7803), with a minimum of 6.2 and a maximum of 9.6. Table 1 presents the descriptive statistics for additional variables examined, including lipid profile components (total cholesterol, HDL-cholesterol, LDL-cholesterol, and triglycerides) and the duration of treatment.
| Variables | Age, (y) | HbA1c | Total Cholesterol | TG | HDL | LDL | Treatment Duration, (mo) |
|---|---|---|---|---|---|---|---|
| No. | |||||||
| Valid | 61 | 61 | 61 | 61 | 61 | 61 | 61 |
| Missing | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Mean | 53.92 | 7.495 | 176.84 | 136.15 | 56.38 | 87.30 | 41.56 |
| Std. Error of Mean | 1.191 | 0.0999 | 5.258 | 5.822 | 1.616 | 3.747 | 2.562 |
| Median | 55.00 | 7.300 | 180.00 | 130.00 | 56.00 | 80.00 | 37.00 |
| Mode | 58 | 8.5 | 180 | 100 | 45 | 80 | 48 |
| Std. Deviation | 9.301 | 0.7803 | 41.066 | 45.470 | 12.622 | 29.267 | 20.008 |
| Variance | 86.510 | 0.609 | 1686.406 | 2067.528 | 159.305 | 856.545 | 400.317 |
| Range | 39 | 3.4 | 206 | 274 | 50 | 165 | 141 |
| Minimum | 35 | 6.2 | 94 | 76 | 30 | 45 | 15 |
| Maximum | 74 | 9.6 | 300 | 350 | 80 | 210 | 156 |
| Sum | 3289 | 457.2 | 10787 | 8305 | 3439 | 5325 | 2535 |
| Percentiles | |||||||
| 25 | 47.50 | 6.900 | 152.00 | 100.50 | 46.00 | 71.00 | 30.00 |
| 50 | 55.00 | 7.300 | 180.00 | 130.00 | 56.00 | 80.00 | 37.00 |
| 75 | 60.00 | 8.000 | 195.00 | 152.00 | 66.00 | 95.50 | 48.00 |
Abbreviations: HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
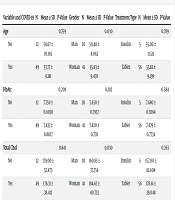
The statistical analysis revealed no significant differences between the COVID-19-positive and COVID-19-negative groups regarding age (P = 0.759), HbA1c level (P = 0.209), total cholesterol level (P = 0.841), triglyceride levels (P = 0.876), HDL (P = 0.504), LDL (P = 0.191), or the duration of diabetes treatment (P = 0.779). However, there was a significant difference in patient age (P = 0.030) and total cholesterol level (P = 0.030) between the male and female groups, with women having higher average age and total cholesterol levels compared to men. No significant differences were observed between the two gender groups for the other variables examined, including HbA1c level (P = 0.282), triglyceride level (P = 0.518), HDL (P = 0.692), LDL (P = 0.085), and duration of diabetes treatment (P = 0.227).
Additionally, the analysis found no significant differences between the groups receiving oral medication and those receiving insulin in terms of age (P = 0.789), HbA1c level (P = 0.584), total cholesterol level (P = 0.263), triglyceride levels (P = 0.162), HDL (P = 0.718), LDL (P = 0.463), or duration of diabetes treatment (P = 0.545) (Table 2).
| Variable and COVID-19 | N | Mean ± SD | P-Value | Gender | N | Mean ± SD | P-Value | Treatment Type | N | Mean ± SD | P-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0.759 | 0.030 | 0.789 | ||||||||
| No | 12 | 54.67 ± 10.165 | Man | 20 | 50.40 ± 8.062 | Insulin | 5 | 55.00 ± 11.511 | |||
| Yes | 49 | 53.73 ± 9.181 | Woman | 41 | 55.63 ± 9.470 | Tablet | 56 | 53.82 ± 9.199 | |||
| HbA1c | 0.209 | 0.282 | 0.584 | ||||||||
| No | 12 | 7.750 ± 0.6038 | Man | 20 | 7.650 ± 0.7957 | Insulin | 5 | 7.680 ± 0.9284 | |||
| Yes | 49 | 7.433 ± 0.8107 | Woman | 41 | 7.420 ± 0.7711 | Tablet | 56 | 7.479 ± 0.7734 | |||
| TotalChol | 0.841 | 0.030 | 0.263 | ||||||||
| No | 12 | 179.00 ± 52.475 | Man | 20 | 160.85 ± 37.754 | Insulin | 5 | 157.00 ± 61.604 | |||
| Yes | 49 | 176.31 ± 38.412 | Woman | 41 | 184.63 ± 40.733 | Tablet | 56 | 178.61 ± 39.048 | |||
| TG | 0.876 | 0.518 | 0.162 | ||||||||
| No | 12 | 138.00 ± 51.384 | Man | 20 | 130.70 ±56.230 | Insulin | 5 | 108.80 ± 30.261 | |||
| Yes | 49 | 135.69 ± 44.478 | Woman | 41 | 138.80 ± 39.716 | Tablet | 56 | 138.59 ± 45.987 | |||
| HDL | 0.504 | 0.692 | 0.718 | ||||||||
| No | 12 | 58.58 ± 10.544 | Man | 20 | 55.45 ± 10.435 | Insulin | 5 | 54.40 ± 12.260 | |||
| Yes | 49 | 55.84 ± 13.120 | Woman | 41 | 56.83 ± 13.660 | Tablet | 56 | 56.55 ± 12.746 | |||
| LDL | 0.191 | 0.085 | 0.463 | ||||||||
| No | 12 | 77.33 ± 20.628 | Man | 20 | 78.05 ± 18.911 | Insulin | 5 | 96.60 ± 65.972 | |||
| Yes | 49 | 89.73 ± 30.697 | Woman | 41 | 91.80 ± 32.425 | Tablet | 56 | 86.46 ± 24.684 | |||
| Treatment Time | 0.779 | 0.227 | 0.545 | ||||||||
| No | 12 | 40.08 ± 9.861 | Man | 20 | 37.10 ± 9.558 | Insulin | 5 | 46.80 ± 23.167 | |||
| Yes | 49 | 41.92 ± 21.869 | Woman | 41 | 43.73 ± 23.287 | Tablet | 56 | 41.09 ± 19.873 |
Abbreviations: HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
The Pearson correlation coefficient test revealed a significant positive relationship between HbA1c and triglyceride levels (P = 0.016). A significant association was also found between total cholesterol and triglyceride levels (P = 0.000), as well as between total cholesterol and LDL levels (P = 0.000). Furthermore, a positive correlation was observed between LDL and triglyceride levels (P = 0.033) (Table 3).
| Variable | Age, (y) | HbA1c | Total Chol | TG | HDL | LDL | Treatment Duration, (mo) |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| Pearson correlation | 1 | -0.027 | -0.073 | -0.155 | 0.116 | -0.116 | 0.186 |
| Sig. (2-tailed) | 0.838 | 0.578 | 0.232 | 0.372 | 0.373 | 0.151 | |
| N | 61 | 61 | 61 | 61 | 61 | 61 | 61 |
| HbA1c | |||||||
| Pearson correlation | -0.027 | 1 | 0.186 | 0.308 a | -0.014 | 0.115 | 0.056 |
| Sig. (2-tailed) | 0.838 | 0.150 | 0.016 | 0.918 | 0.378 | 0.667 | |
| N | 61 | 61 | 61 | 61 | 61 | 61 | 61 |
| Total Chol | |||||||
| Pearson correlation | -0.073 | 0.186 | 1 | 0.805 b | -0.015 | 0.444 b | 0.021 |
| Sig. (2-tailed) | 0.578 | 0.150 | 0.000 | 0.911 | 0.000 | 0.670 | |
| N | 61 | 61 | 61 | 61 | 61 | 61 | 61 |
| TG | |||||||
| Pearson correlation | -0.155 | 0.308 a | 0.805 b | 1 | 0.026 | 0.273 a | -0.043 |
| Sig. (2-tailed) | 0.232 | 0.016 | 0.000 | 0.840 | 0.033 | 0.741 | |
| N | 61 | 61 | 61 | 61 | 61 | 61 | 61 |
| HDL | |||||||
| Pearson correlation | 0.116 | -0.014 | -0.015 | 0.026 | 1 | -0.177 | 0.109 |
| Sig. (2-tailed) | 0.372 | 0.918 | 0.911 | 0.840 | 0.173 | 0.401 | |
| N | 61 | 61 | 61 | 61 | 61 | 61 | 61 |
| LDL | |||||||
| Pearson correlation | -0.116 | 0.115 | 0.444 b | 0.273 a | -0.177 | 1 | 0.212 |
| Sig. (2-tailed) | 0.373 | 0.378 | 0.000 | 0.033 | 0.173 | 0.102 | |
| N | 61 | 61 | 61 | 61 | 61 | 61 | 61 |
| Treatment time | |||||||
| Pearson correlation | 0.186 | 0.056 | 0.021 | -0.043 | 0.109 | 0.212 | 1 |
| Sig. (2-tailed) | 0.151 | 0.667 | 0.870 | 0.741 | 0.401 | 0.102 | |
| N | 61 | 61 | 61 | 61 | 61 | 61 | 61 |
Abbreviations: HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
a Significant at 0.05.
b Significant at 0.01.
5. Discussion
COVID-19 has exhibited a wide range of severity, from individuals showing no symptoms to those experiencing severe illness that can result in death (17). Adverse outcomes have been associated with existing chronic conditions, such as high blood pressure and diabetes (18). Diabetes mellitus (DM) is widely recognized as a significant comorbidity among patients diagnosed with COVID-19, due to its high prevalence (19). Individuals with diabetes are more vulnerable to infections and are at a higher risk of experiencing a negative prognosis compared to non-diabetic individuals (20). During the lockdown period, numerous studies have documented a decline in glycemic control among individuals diagnosed with COVID-19 (21, 22). This study focuses on the clinical features and outcomes of diabetic patients in Semnan during the COVID-19 crisis. We selected the Endocrinology Clinic over a hospital setting to collect data, as the clinic focuses on routine outpatient diabetes management, making it more representative of real-world care during the COVID-19 pandemic. Hospital data, especially from acute-care settings, might have skewed the results toward more severe cases. Our study concentrated on long-term glycemic control using HbA1c, as it was the most consistently available and clinically relevant parameter in the collected data.
The findings from our study suggest that there were no significant differences in glycemic control, as measured by HbA1c levels, between diabetic individuals infected with COVID-19 and those who were not infected. These results are consistent with another study, which indicated that COVID-19 did not have a notable effect on blood sugar regulation in individuals diagnosed with type 2 diabetes (23). However, several studies present findings that contradict the results obtained in our study. Multiple investigations on glycemic management in people with type 2 diabetes during the lockdown period revealed elevated levels of HbA1c and average glucose readings (24). A retrospective cohort analysis demonstrated that the average HbA1c level increased from 6.9% in 2019 to 7.2% in 2020, suggesting a decline in glycemic control during the pandemic (25). Another study found that HbA1c levels significantly increased during the pandemic compared to pre-pandemic values, with a notable rise in glucose levels as well (26).
The dysregulation of glycemic control can be attributed to shifting lifestyle patterns, such as increased consumption of sugary foods and snacks, reduced physical activity, and excessive sedentary behavior (8), limited availability of healthcare services during the pandemic, and the psychological stress associated with the pandemic (27), all of which may have led to changes in body composition. On the other hand, some studies have shown a notable improvement in glycemic values during the COVID-19 outbreak compared to levels before the pandemic (28). This improvement could be attributed to patients’ personal care routines, including access to various educational resources that facilitated positive lifestyle changes, the ability to work remotely, which resulted in better eating habits (e.g., consistent meal times and homemade meals), and dedicated time for regular physical exercise (28).
The variation in the outcomes of our study compared to other studies may be due to several factors. Our sample size consisted of 64 participants, which is relatively small. In comparison to other studies that have reported significant changes in glycemic control during the pandemic, our smaller sample size might have limited our ability to detect statistically significant differences. Additionally, it is important to note that our study participants may have had distinct characteristics compared to those in previous studies, particularly in terms of the severity of their illness or their adherence to diabetes management. If our population had generally better-controlled diabetes or exhibited higher levels of adherence to their treatment plans, this could have masked any potential negative effects of COVID-19 on blood sugar management, which were observed in other studies. Moreover, the behavioral adjustments made during the pandemic, such as changes in diet or increased physical activity, might have also contributed to maintaining glycemic control in our study population. These lifestyle modifications may not have been as prevalent in the populations of other studies, which could explain the observed differences in outcomes.
The study also found no significant differences in lipid measurements, such as total cholesterol, HDL cholesterol, LDL cholesterol, and triglyceride levels, between the two groups (diabetic patients infected with COVID-19 and those who are not). However, this contradicts the results of similar studies that have reported changes in lipid profiles among individuals with varying levels of COVID-19 illness (29-31). A single-center study in India reported that during the COVID-19 pandemic, total cholesterol decreased from 178.1 ± 40.8 mg/dL to 170.5 ± 38.4 mg/dL (P < 0.05), while HDL cholesterol and triglyceride levels remained stable, showing no significant changes. LDL cholesterol showed a non-significant decrease from 101.1 ± 34.1 mg/dL to 95.5 ± 35.0 mg/dL (P = 0.07) (32).
Our data analysis also revealed a significant direct relationship between HbA1c concentrations and triglyceride levels. This correlation between glycemic control and lipid metabolism aligns with the findings of other studies, which indicate that poor glycemic control often leads to dyslipidemia in people with type 2 diabetes (33).
Additionally, the study identified significant differences in age and total cholesterol levels between male and female subjects, with diabetic women showing higher average ages and total cholesterol levels compared to diabetic men. Although our study includes comparisons of biochemical parameters between men and women, as well as between insulin and non-insulin treatment groups, the primary focus remains on comparing COVID-19-infected and non-infected groups. The additional comparisons were included to explore potential gender-specific differences and treatment variations in diabetes management. However, we acknowledge that age and hormonal differences, particularly among women, may act as confounding factors, potentially influencing lipid and glycemic outcomes. Future studies with more controlled designs, stratifying by age and hormonal status, are needed to better clarify the gender-related differences observed in our results.
The higher average age of the diabetic women in the study can be explained by the fact that diabetes tends to peak in men at an earlier age (65 - 69 years) compared to women (70 - 79 years) (34). Similarly, women generally exhibit higher levels of all lipid profile measures, except HDL, compared to men (35), which aligns with the findings of the present study.
Finally, the research revealed no significant differences in the analyzed factors between individuals prescribed oral medications and those undergoing insulin therapy. Given that HbA1c serves as a measure of blood sugar regulation over an extended period (36), it is reasonable to infer that both therapeutic approaches have shown similar efficacy in managing patients' long-term blood sugar levels.
5.1. Limitations
One notable limitation of this research is the lack of data regarding the glycemic and metabolic management of individuals who did not seek assistance from the Endocrinology and Metabolism Clinic at the Kowsar Education, Research, and Treatment Center during the COVID-19 outbreak. This lack of information may impact the outcomes of the study. Additionally, the sample size of the study is relatively small, which could limit the generalizability of the results. Further investigation using larger, longitudinal research methodologies is needed to conclusively assess the long-term effects of COVID-19 infection on blood sugar regulation and lipid parameters among individuals with type 2 diabetes.
5.2. Conclusions
The findings of this research indicate that there were no significant differences in glycemic control, as measured by HbA1c levels, between diabetic individuals who contracted COVID-19 and those who did not. However, there were notable differences in age and total cholesterol levels between male and female diabetic patients, with women being older and having higher total cholesterol levels than men. Additionally, no significant distinctions were observed between patients on oral medication and those receiving insulin treatment. The study also identified positive correlations between HbA1c and triglyceride levels, emphasizing the importance of continuous diabetes management that addresses both glycemic and lipid-related factors.
.png)