1. Background
The antibacterial property of a substance refers to its ability to inhibit the growth, reproduction, or survival of bacteria. An antibacterial agent effectively reduces or eliminates bacterial infections, making it crucial in various fields, particularly medicine, where these materials prevent or treat bacterial infections in wounds, implants, medical devices, and other applications. Understanding the types of bacteria and their mechanisms of destruction is essential for developing effective antibacterial materials.
Bacteria are classified based on different criteria, such as shape, Gram staining, and oxygen demand, among others. One of the most important classifications divides bacteria into Gram-positive and Gram-negative categories based on Gram staining. Gram-positive bacteria, such as Staphylococcus aureus, have a thick peptidoglycan layer in their cell walls that retains the crystal violet stain, appearing purple under a microscope. In contrast, gram-negative bacteria, such as Escherichia coli, have a thinner peptidoglycan layer and do not retain the crystal violet stain, appearing pink or red under a microscope. This study focuses on these two classes of bacteria (1-6). Antibacterial agents work through various mechanisms, such as controlling cell wall synthesis, which is essential for maintaining bacterial structural integrity; inhibiting protein synthesis by targeting ribosomes, which are crucial for translating mRNA into proteins; disrupting nucleic acid synthesis; impairing cell membrane function; or inhibiting ATP synthase. Each mechanism disrupts critical bacterial processes, leading to bacterial death or inhibition of growth (7-10).
Antibacterial agents are utilized across numerous fields to prevent or treat bacterial infections. Key applications include healthcare (e.g., medical devices, wound care, and sterilization), pharmaceutical production, personal care products, the food industry, agriculture, water treatment, and textiles. In medical applications, where devices and materials come into direct contact with the human body, the antibacterial property is especially critical. Antibacterial agents help reduce microbial infections in medical devices and implants, particularly for immunocompromised patients. They combat antibiotic resistance, minimize antibiotic use, improve postoperative care, and enhance the longevity of medical devices (11-16).
Biomaterials are essential in biomedical applications, including implants, drug delivery systems, and tissue engineering, due to their biocompatibility and potential to restore physiological functions and improve patients' quality of life. They can be derived from natural sources or synthesized in laboratories. Despite their promise, unresolved issues such as infection persist (11, 17, 18). Biocomposites, which combine biopolymers and reinforcing agents, are particularly promising for medical applications. Their unique biological and mechanical properties make them suitable for tissue engineering, regenerative medicine, and implantable devices, with potential uses in other medical fields (19).
Biocomposites are eco-friendly, non-toxic, non-carcinogenic, and non-inflammatory. They offer mechanical strength and elasticity to handle physiological loads and are biodegradable, making them useful for temporary implants and drug delivery systems. Antibacterial properties are crucial for the development of antibacterial composites for prostheses, artificial compartments, and replacements, ensuring functionality while maintaining human tissue integrity (11, 19).
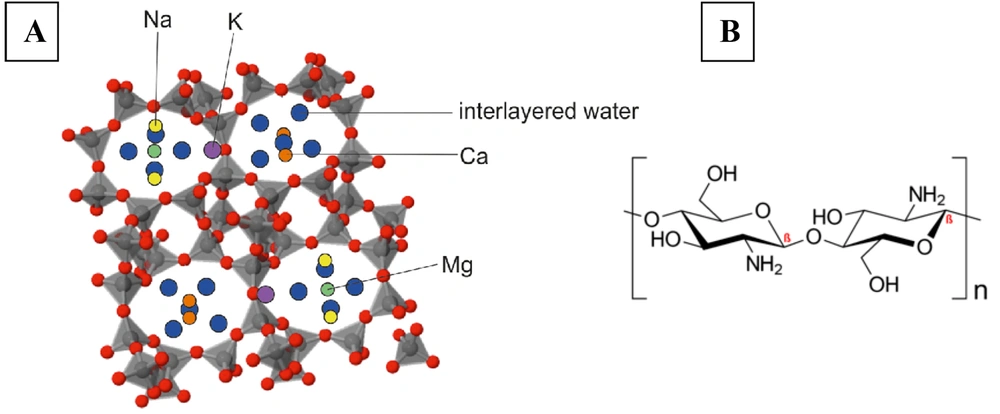
Two notable antibacterial biomaterials are chitosan and zeolites. Chitosan is a natural biopolymer of marine origin derived from chitin, which is found in the exoskeletons of organisms such as lobsters, crabs, and shrimps. It is an amino polysaccharide obtained through the deacetylation of chitin, characterized by its biocompatibility, bioactivity, and non-toxicity. Chitosan also has antioxidant and antibacterial properties, making it ideal for tissue engineering. It regulates coagulation, inhibits inflammatory mediators, and promotes faster healing (15, 20).
Zeolites, found in both natural and synthetic forms, are minerals with an ordered porosity structure and a microporous pore range. Zeolite nanoparticles differ from other mesoporous nanoparticles due to their unique structure and favorable physicochemical properties, such as lower cytotoxicity, higher loading capacity, and enhanced intracellular targeting specificity and efficiency. These characteristics give zeolites superior biocompatibility and biomolecular delivery capacity. Consequently, zeolites have wide-ranging biomedical applications, including as MRI contrast agents, antibacterial agents, antitumor adjuvants, antidiarrheal agents, and in bone formation studies, Alzheimer’s disease research, hemodialysis, drug delivery, and dental applications (21-23).
The specific structures and properties of these materials are further detailed in the following section. In modern clinical applications, there is a significant need to design and develop wound dressings with high antibacterial and procoagulant activity to achieve effective wound healing (24, 25). In one study, Wang et al. developed a gauze containing zeolite, calcium, and copper. The CaCu-ZG gauze demonstrated excellent blood-clotting and antibacterial properties, proving to be safe for internal use. Compared to standard medical gauze, CaCu-ZG significantly accelerated blood clotting and improved bacterial elimination in both in vitro and in vivo experiments. The gauze's wound-healing efficacy was validated in a mouse model, highlighting its potential for medical wound treatment applications (26).
Researchers have also found that metal particles supported on zeolites can effectively combat microorganisms while exhibiting low overall toxicity (27). A method using sound waves to deposit metals has gained traction due to its efficiency, cost-effectiveness, and environmental benefits. In a recent study, De León Ramirez et al. explored the antimicrobial properties of zinc particles supported on LTA zeolite through a sound-wave-assisted method. They discovered that at a concentration of 30 mg/mL, drying the ZnO2@NaA material activated a mechanism that completely inhibited the growth of Enterococcus faecalis. This material also demonstrated antifungal potential, inhibiting Candida albicans growth by 90% at a concentration of 1 mg/mL. Various analytical techniques, including XRD, SEM, and FTIR, confirmed the presence of ZnO2 nanoparticles in the composite (28). Additionally, numerous studies have investigated zeolite and zeolite-supported metal ions for antibacterial applications (29-33).
Regarding chitosan’s antibacterial effects, Sousa et al. examined the cytotoxic and antibacterial properties of chitosan and its derivatives modified with ethylenediamine and phthalic anhydride. The results showed that both hydrophobic and hydrophilic chains enhanced chitosan's antibacterial properties, increasing its applicability in medical and pharmaceutical sciences. These materials exhibited inhibitory effects exceeding 70% against S. aureus and E. coli. Tests with human fibroblast cells using the bromide reduction method showed no toxicity (34). Similarly, Wang et al. investigated chitosan derivatives containing six-membered rings for their antibacterial and anti-biofilm properties against harmful bacteria such as E. coli and S. aureus. They found that increasing the water-repellence, alkalinity, and electrical charge of these derivatives significantly enhanced their ability to kill bacteria and prevent biofilm formation, even though unmodified chitosan showed limited antibacterial efficacy (35). Numerous additional studies have focused on this area in recent years (36-38).
Interestingly, the combined effect of zeolite and chitosan has not been extensively studied.
2. Objectives
Therefore, this research aims to synthesize a biocomposite using chitosan and clinoptilolite zeolite and evaluate its antibacterial properties. To achieve this, a synthesis method was designed, maintaining a constant chitosan concentration while varying clinoptilolite zeolite concentrations at three levels. The biocomposite was prepared, cut, and sterilized using UV light. Its antibacterial properties were then tested against gram-negative E. coli and gram-positive S. aureus.
3. Methods
For this study, natural zeolite (Clinoptilolite) was supplied by Niakan Company (Semnan, Iran), and chitosan with medium molecular weight and acetic acid was purchased from Merck Company (Germany).
Zeolites are crystalline metal oxides composed of tetrahedral atoms (e.g., Si, Al, P, Ga, B, Ti, Ge, Fe) connected to four or two oxygen atoms. Each oxygen atom is bonded to two atoms that form a tetrahedron. These porous structures have a general chemical formula as follows:
In the zeolite structure, "M" represents an alkali or alkaline earth cation with valence (n), "w" is the number of water molecules, and "x" and "y" denote the molar concentrations of tetrahedra. Typically, the (y/x) ratio ranges between 1 and 5 but can increase up to 100 in siliceous zeolites (21-23).
Zeolites employ various antimicrobial mechanisms to eliminate microbes, including physical adsorption, ion exchange, and indirect catalysis. During physical adsorption, zeolites physically capture microbes on their surface, potentially immobilizing and killing them. Through ion exchange, zeolites can exchange their structural ions with antimicrobial metals like silver or copper, releasing these ions into the environment to damage microbial cell walls and membranes. Additionally, zeolites can produce reactive oxygen species (ROS), such as hydrogen peroxide, which can harm microorganisms (39).
The biocomposite synthesis procedure is as follows: In the first step, clinoptilolite particles are impregnated in distilled water for 24 hours and then dried at 110°C for 3 hours. In the second step, chitosan is dissolved in acetic acid (diluted, 2% w/v) and allowed to rest overnight. Subsequently, dried zeolite at varying concentrations is added to the acetic acid solution to prepare compositions with 0.5%, 1%, and 2% weight. Finally, the chitosan gel and zeolite suspension are mixed. The final mixture is placed in molds and transferred to a freeze dryer (at -80°C for 24 hours).
The sample coded as (0S) corresponds to the composition containing 0.5% zeolite that has not been exposed to UV sterilization. Similarly, sample (S1S) refers to the sample with 0.5% zeolite, (S2S) to the sample with 1% zeolite, and (S3S) to the sample with 2% zeolite, all of which have been sterilized using UV radiation.
3.1. Antibacterial Assay
For this assay, the disk diffusion method (DDM) was used. The medium utilized in this method was nutrient agar with a pH range of 7.2 to 7.4. To assess the antibacterial activity of the zeolite-chitosan biocomposite, the materials were tested against gram-positive bacteria (S. aureus) and gram-negative bacteria (E. coli). After preparing a 0.5 McFarland standard, one or two colonies of bacteria grown on nutrient agar were picked up with a sterile loop under a biological hood and dissolved in sterile physiological serum in a test tube. The turbidity of the solution was then compared with the 0.5 McFarland standard. Once the turbidity of the bacterial suspension in the physiological serum matched the 0.5 McFarland standard, the solution was mixed using a sterile swab. After draining excess liquid (by pressing the swab firmly against the side of the tube to remove the liquid), the suspension was spread evenly over the surface of a Petri dish containing nutrient agar using the lawn culture method.
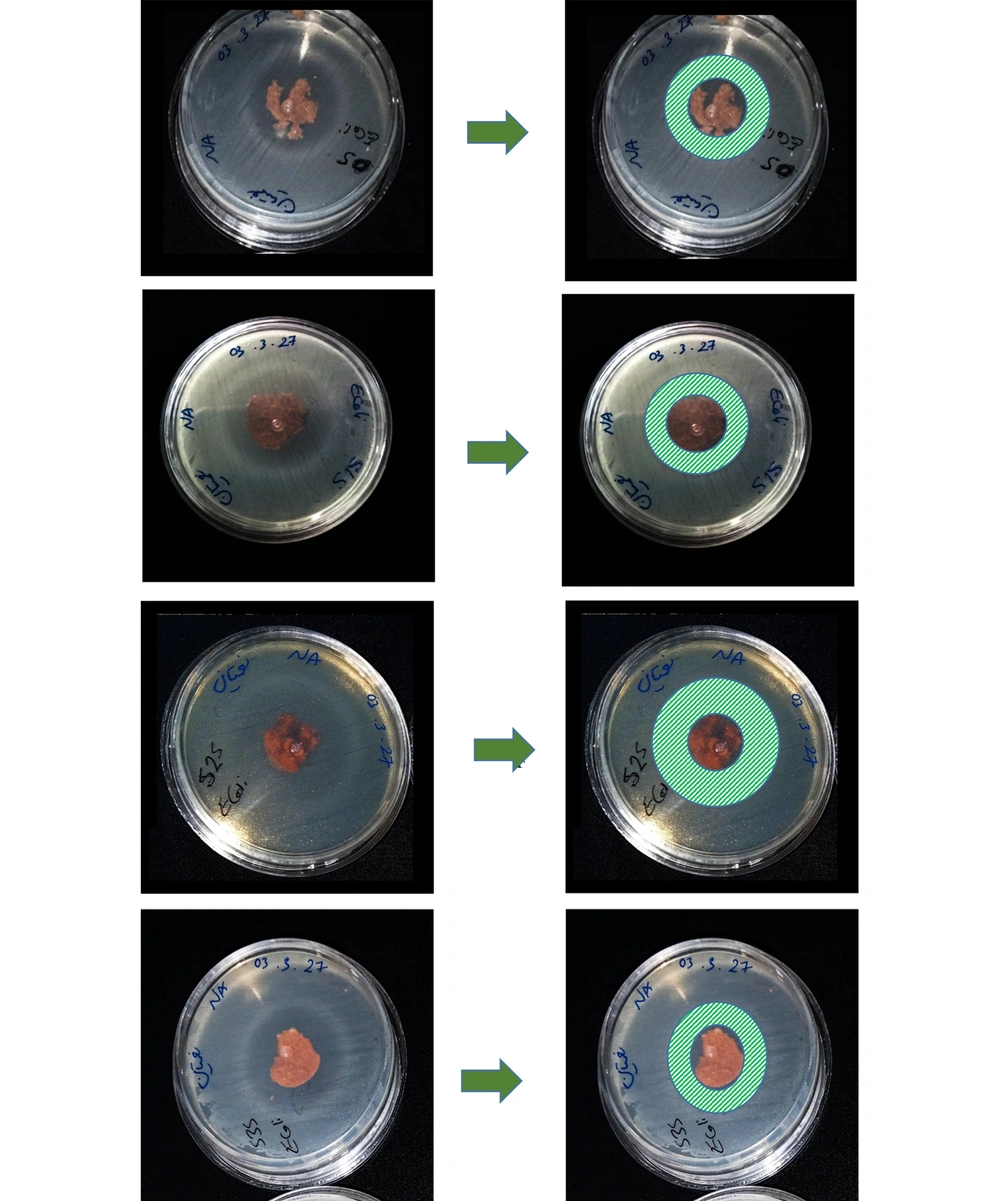
To examine the antibacterial properties of the biocomposite structure, it was first cut into equal circular pieces. The samples were then placed 5 cm away from a UV light source and exposed to ultraviolet radiation for 20 minutes before being placed on the culture medium at intervals. Sterile disks containing zeolite and chitosan were arranged on the agar in a circular pattern. The cover of the Petri dish was closed for 15 minutes to allow the tablets to adhere to the agar. Subsequently, the dish was placed in an incubator at 37°C for 24 hours. After this incubation period, the size of the clear zones (inhibition halos) surrounding the tablets was measured using reflected light (Figure 1) (40, 41).
4. Results
4.1. Characterization of Biocomposite Structure
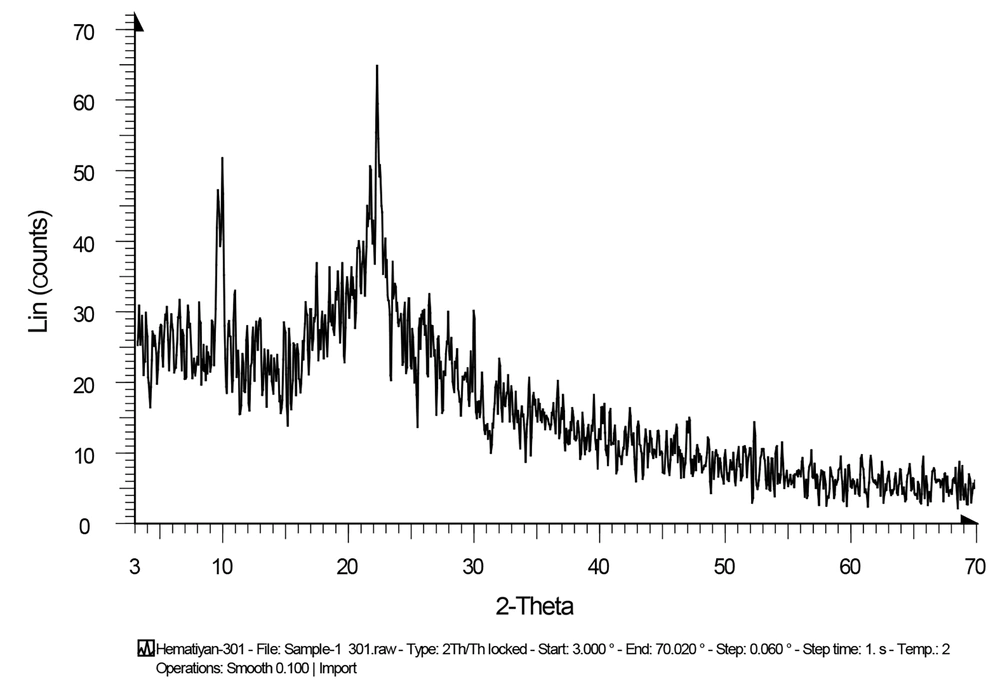
The crystallinity of the biocomposite was estimated using an XRD diffractometer (D8-ADVANCE Bruker, 40 kV, 20 mA, Cu Kα radiation). The XRD pattern for the synthesized biocomposite containing clinoptilolite and chitosan is shown in Figure 2. The diffraction peaks observed in the figure align well with the structure of clinoptilolite (JCPDS: 39-1383).
The sharp and strong diffraction peaks in the figure indicate that the product is well-crystallized. The X-ray diffraction analysis of the natural zeolite revealed that it was primarily composed of crystalline phases of clinoptilolite, with minor amounts of other minerals such as aluminum silicate. Additional peaks corresponding to chitosan were also observed. The X-ray diffraction pattern of pure chitosan exhibited two distinct peaks at 2θ angles of 10 and 20 degrees, indicating a well-organized crystalline arrangement. The intensity of these peaks suggested that the material was highly crystalline with minimal amorphous content (42, 43).
4.2. Antibacterial Effect
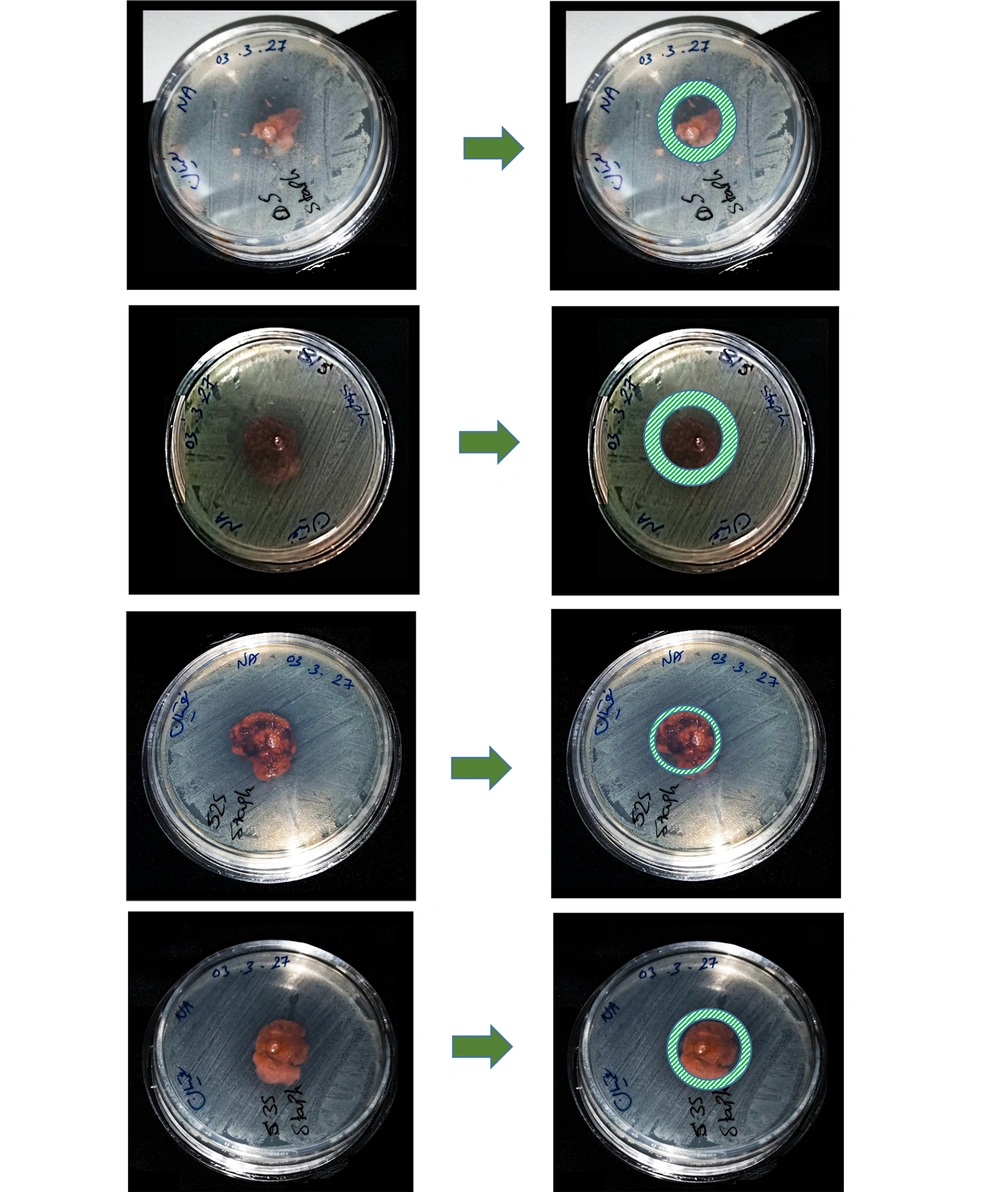
As explained previously, in the initial step, a biocomposite containing chitosan biopolymer and zeolite bioceramic was synthesized. In this composite, the amount of chitosan was kept constant, while the amount of zeolite was varied. The results of the antibacterial test demonstrated zones of inhibition. These zones showed better results against gram-negative bacteria (E. coli). However, against gram-positive bacteria (S. aureus), the results were less prominent. Although zones of inhibition were observed, their diameters were not very large.
It is noteworthy that, due to the spongy nature of the produced biocomposite, the cut tablets were not completely circular, resulting in slightly irregular shapes. Therefore, the results may not be fully comparable, but it is evident that the antibacterial properties of the biocomposite are more significantly attributed to the chitosan component than to the zeolite. In future experiments, it would be best to adjust the experimental conditions so that all variables except the zeolite concentration remain constant, enabling more accurate comparisons of findings (Figure 3 and Figure 4).
As mentioned earlier, the effect of the prepared biocomposite against S. aureus was not very strong, suggesting that other gram-positive bacteria could be tested in future studies. Additionally, since the first sample was placed in the culture medium without UV sterilization and still showed good resistance against E. coli, it can be inferred that this biocomposite exhibits bactericidal properties.
5. Discussion
Antibacterial agents: a) can reduce microbial infections. Medical devices and implants are particularly vulnerable to bacterial colonization, which can result in severe infections. These agents control bacterial growth and prevent the spread of infections, which is especially critical for vulnerable patients; b) can eliminate the need for antibiotics and help combat antibiotic resistance; c) can improve the treatment process by preventing infections at the site of an implant or wound; d) can increase the longevity of implants and devices. Bacterial activity can damage implants or artificial devices (12, 14). Therefore, this category of materials is of paramount importance.
Antibacterial agents are classified into three types based on their mechanisms of bacterial eradication. Class, one involves direct bactericidal action, such as releasing ions or producing ROS that destroy bacterial cell walls. Class two includes antibiofouling agents, which resist bacterial adhesion and prevent biofilm formation. Class three encompasses chemical functionalization, where materials are coated or impregnated with antibacterial agents, or their pH is altered to inhibit bacterial growth (7, 11, 44-46).
Most research to date has focused on synthetic materials loaded with appropriate ions. However, the creation of synthetic materials is often a challenging and costly process (28, 45). As highlighted in the results section, the materials used in this study are natural and possess intrinsic antibacterial properties. The method developed in this research has resulted in the production of biocomposites that demonstrate significant resistance to the tested bacteria, even without sterilization. This biocomposite represents a promising material for widespread medical applications.
5.1. Conclusions
Due to the wide application of antibacterial compounds in various fields, particularly in medicine, and their extensive use in biomedicine, their synthesis has become essential. Antibacterial compounds intended for use within the body must be biocompatible and biodegradable, necessitating the use of various biomaterials. Previous research has demonstrated that chitosan and zeolite each possess favorable properties individually. Therefore, this research investigated the synthesis of a zeolite-chitosan composite and studied its antibacterial properties. The results indicated that this biocomposite exhibits significant resistance against E. coli bacteria but is less effective against Staphylococcus bacteria. Additionally, as the synthetic biocomposites were composed of a fixed amount of chitosan combined with varying amounts of zeolite, the findings revealed that chitosan is more effective than zeolite in imparting antibacterial properties. Moreover, altering the amount of zeolite did not significantly enhance antibacterial efficacy. However, this aspect should be further examined, particularly at higher concentrations of zeolite.