1. Background
Lung cancer is one of the leading causes of cancer morbidity and mortality worldwide, with its epidemiology marked by a higher incidence in smokers and individuals exposed to environmental carcinogens (1). The complexity of lung cancer, characterized by its heterogeneous nature and resistance to conventional therapies, necessitates the search for innovative therapeutic agents that can effectively target tumor cells while minimizing harmful effects. The etiology of lung cancer is multifactorial; while smoking is the strongest risk factor, it often acts synergistically with other factors. Smokers exposed to additional risk factors, such as radon and asbestos, face an increased risk of developing lung cancer (2). Not all smokers develop lung cancer, highlighting the role of other factors, such as genetics, in influencing susceptibility. Although various treatment modalities, including surgery, radiation, chemotherapy, and immunotherapy, are available, the pursuit of safer and more effective approaches remains a priority. Traditional chemotherapy drugs are challenged by issues of resistance, systemic toxicity, and limited bioavailability (3). In advanced stages, novel treatment approaches, such as targeted therapies, are also available. The choice of treatment approach, whether used individually or in combination, depends on the patient's specific condition (4).
Researching new chemotherapy drugs to improve effectiveness is essential. Natural active compounds are regarded as a rich source of potential new anticancer agents. Investigating the anticancer potential of these natural ingredients and their application in cancer drug development remains a key research focus (3, 5).
Chrysin (5,7-dihydroxyflavone) (Chr), a polyphenolic flavone commonly found in fruits, vegetables, and honey, is known for its roles in various biological pathways, including the prevention, delay, or reversal of cancer development (6). It possesses anti-inflammatory, antioxidant (7), immunomodulatory (8), and anticancer properties (9). Chrysin and its derivatives have shown significant apoptotic and anti-proliferative effects in human cancer cells from various origins, notably lung (10), breast (11), prostate (12), and colorectal cancers (13).
2. Objectives
In this study, we report the in vitro evaluation of Chr’s anticancer properties. Additionally, Chr’s capacity to inhibit cell growth and induce apoptosis was assessed in the A549 lung cancer cell line. This study underscores the potential of Chr to enhance the pharmacological efficacy of natural compounds, paving the way for their broader therapeutic application.
3. Methods
3.1. Materials
All reagents and materials were used as received. Human A549 lung cancer cells were obtained from the National Cell Bank of Iran (NCBI; Pasteur Institute, Tehran, Iran). Dimethyl sulfoxide (DMSO), Dulbecco’s Modified Eagle’s Medium (DMEM), 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), Phosphate Buffered Saline (PBS), Fetal Bovine Serum (FBS), Penicillin-Streptomycin (Pen/Strep), and Trypsin-EDTA were sourced from Gibco.
3.2. Cell Culture
A549 cells were cultured in DMEM medium supplemented with 10% fetal bovine serum, 1% penicillin, and 1% streptomycin. Cells were maintained under controlled culture conditions at 37ºC with 5% CO₂ and 95% humidity.
3.3. In Vitro Cytotoxicity
Cell viability following Chr treatment was assessed using the MTT assay in A549 cancer cells as described by Khoshravan et al. (14). A549 cancer cells were seeded in a 96-well plate at a density of 1 × 104 cells per well and incubated at 37ºC for 24 hours. The cells were then treated with 100 μL of medium containing various concentrations of Chr (0, 5, 10, 20, and 40 μM) and incubated at 37ºC for 24, 48, and 72 hours. Quantitative measurement of cell survival was conducted using the MTS kit, and the formazan salts formed were measured using an EL X 800 microplate absorbance reader (BioTek Instruments, Winooski, VT) at 570 nm.
3.4. Gene Expression of Apoptosis Related Genes
To study the expression of apoptosis-related genes in cancer cells, 24 hours after incubation with different concentrations of Chr, the culture medium was completely removed, and the cells were rinsed with PBS. The cells were then detached, and RNA was extracted using the RNX-Plus kit according to the manufacturer’s instructions. The concentration and purity of the extracted RNA were determined using a spectrophotometer (Thermo Scientific™ NanoDrop™) and confirmed by agarose gel electrophoresis. Total RNA was reverse-transcribed into cDNA using the Prime Script RT reagent kit, following the manufacturer’s protocol. Real-time PCR was conducted with SYBR Green PCR Master Mix (Ampliqon, Denmark) to analyze the expression levels of Bax, Bcl2, and human telomerase reverse transcriptase (hTERT). The expression of these target genes was compared to the housekeeping gene GAPDH using the comparative threshold cycle (Ct) method.
3.5. Statistical Analysis
The studies were conducted in triplicate, and the results are presented as the mean ± standard deviation. Data were analyzed for statistical significance using Student's t-test. A significance level of 0.05 was applied, with P < 0.05 indicating significance (*) and P > 0.05 indicating non-significance (ns).
4. Results
4.1. Cytotoxicity Assay
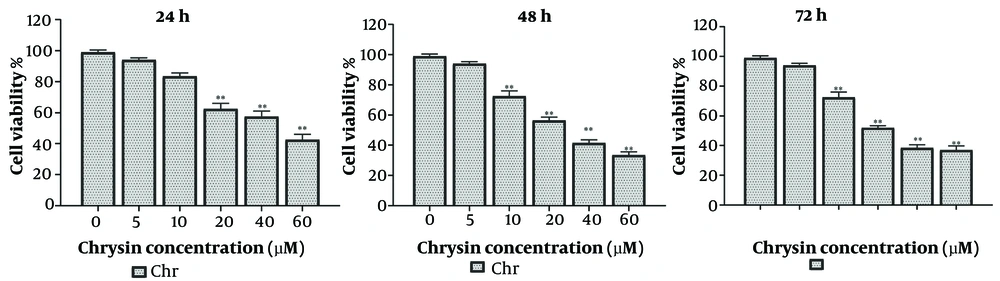
The cytotoxic potential of Chr against malignant cells was assessed through an MTT assay conducted on A549 lung cancer cell lines. Following treatment durations of 24, 48, and 72 hours, Chr demonstrated dose-dependent inhibitory effects at concentrations ranging from 0 to 40 μM. The IC50 value of Chr for A549 cell lines was determined to be 20.51 ± 1.27 μM (Figure 1). These findings suggest that Chr exhibits significant cytotoxicity against cancerous cell lines.
4.2. Quantitative Real-time PCR Assay
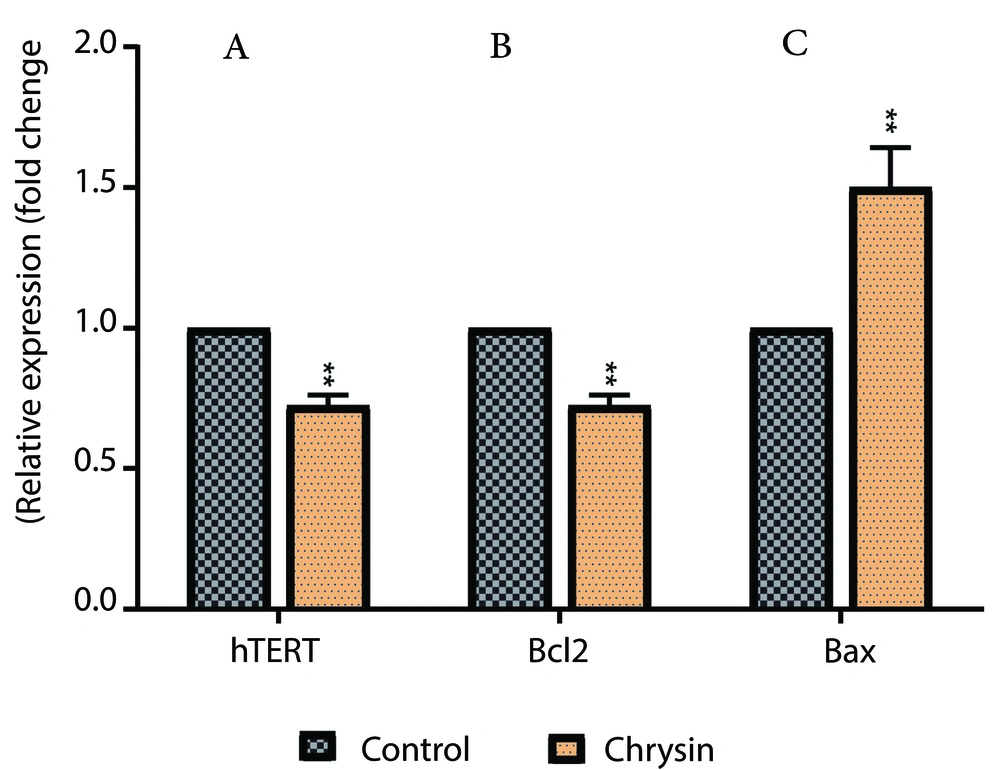
The qPCR assay is a highly sensitive method used to quantify gene expression levels in various biological samples. Analysis of Bcl2 and hTERT gene expression in cancer cells revealed that Chr caused a significant decrease in the expression of these genes compared to the control group (Figure 2). In contrast, Bax gene expression in A549 human lung cancer cells significantly increased in the Chr-treated group compared to the control.
Inhibitory effects of Chrysin (Chr) on expression levels of A, human telomerase reverse transcriptase (hTERT); B, Bcl2; and C Bax in A549 cancer cells. * P < 0.05 and * * P < 0.01 are the statistical difference between the combination form and individual drugs. Data represented are from three independent experiments.
5. Discussion
The current report provides compelling evidence regarding the anticancer efficacy of Chr in A549 lung cancer cells. The study examined both the cytotoxic effects of Chr and its impact on the expression of key apoptotic genes, which play critical roles in the regulation of programmed cell death. The MTT assay determined an IC50 value of 20 μM, indicating that Chr exhibits significant inhibitory effects on cell proliferation at this dose. This result aligns with previous studies suggesting Chr’s potential to exert cytotoxic effects on various cancer cell lines.
Firouzi-Amandi et al. investigated the efficacy of Chr encapsulated in PLGA-PEG nanoparticles for macrophage modulation, demonstrating that this nanoformulated system possesses macrophage repolarization activities (4). In another study, the anticancer activity of honey combined with Chr was assessed, showing a reduction in cell viability in a time- and dose-dependent manner in treated cancer cells (15). Among various cancer models, Chr's effects on preventing and controlling lung cancer growth have been widely evaluated both in vitro and in vivo (6, 9). In our previous studies, Chr combined with curcumin was shown to inhibit the proliferation, invasion, and metastasis of T47D breast cancer cells by downregulating hTERT gene expression (16).
Human telomerase reverse transcriptase is a critical enzyme that plays an essential role in cellular aging and cancer biology. As the catalytic subunit of the telomerase complex, hTERT is responsible for adding telomeric repeats to the ends of chromosomes, countering the progressive shortening of telomeres that occurs during DNA replication. This process is crucial for maintaining chromosomal stability, especially in stem cells and germ cells, which require extensive proliferative capacity (17).
Furthermore, the real-time PCR assessment provided insights into the molecular mechanisms underlying Chr's anticancer properties. Notably, the reduction in hTERT expression is significant, as hTERT is often associated with the "immortal" nature of cancer cells. By reducing hTERT levels, Chr may contribute to restoring normal cellular aging processes, thereby limiting the growth potential of A549 cells. Additionally, the observed decrease in Bcl2, a protein that inhibits apoptosis, alongside an increase in Bax, a protein that promotes apoptosis, suggests that Chr modulates apoptotic signaling pathways. This shift is crucial for enhancing apoptosis in cancer cells, facilitating their targeted elimination.
Previous research has provided strong evidence that an increase in the expression of key pro-apoptotic genes like Bax, along with a decrease in anti-apoptotic genes such as Bcl2, results in reduced survival rates for cancer cells (18, 19). Studies have shown that a 24-hour exposure period is sufficient to observe significant changes in cell viability and gene expression in response to various anticancer treatments. This duration allows for the evaluation of initial cellular responses to the treatment, including alterations in apoptotic pathways and gene regulation (20).
The findings from this study are significant, indicating that Chr may serve as a promising therapeutic option for lung cancer. Its ability to influence apoptosis-related gene expression and promote cell death underscores its potential as a candidate for further exploration in both preclinical and clinical research.
5.1. Conclusions
In conclusion, this study highlights the significant anticancer efficacy of Chr against A549 lung cancer cells. The identified IC50 value of 20 μM demonstrates that Chr effectively inhibits cell proliferation, underscoring its potential as a therapeutic agent. Moreover, the modulation of apoptotic gene expression, particularly the upregulation of Bax and downregulation of hTERT and Bcl2 genes, suggests that Chr induces apoptosis in A549 lung cancer cells. These findings underscore the need for further research to elucidate the mechanisms underlying Chr's effects and its potential clinical applications. Overall, Chr appears to be a promising candidate for the development of innovative cancer treatments, especially for lung cancer. Future studies should explore its efficacy in vivo and examine potential synergistic interactions with existing therapies.