1. Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported in Wuhan, China, at the end of 2019, and the cause of coronavirus disease 2019 (COVID-19) quickly became a global public health concern (1). In February 2020, the first case of COVID-19 was reported in Qom, Iran. Following this, a large number of patients with COVID-19 were rapidly diagnosed across Iran (2).
In patients with SARS-CoV-2, a spectrum of mild to severe respiratory manifestations with a mortality rate of 2% has been described (3). According to the Centers for Disease Control and Prevention (CDC) in April 2020, a high percentage of hospitalized patients with COVID-19 suffered from underlying conditions such as hypertension, obesity, chronic lung disease, diabetes mellitus, and cardiovascular disease (4-6), which are considered major risk factors for exacerbation of COVID-19 (7).
Allergic diseases include a variety of disorders such as asthma, allergic rhinitis (AR), and food allergies (8). Similar clinical symptoms, such as coughing and sneezing in both allergic diseases and COVID-19, make distinguishing between these two diseases challenging (9). The role of respiratory viruses in the exacerbation of asthma and the varying prevalence rates of asthma in individuals with COVID-19, ranging from < 1% in China to 14% in the USA, have been highlighted in other studies (10, 11). Moreover, a systematic review suggested a potential link between long-COVID symptoms (LC) and allergies (12).
Research increasingly points to the possibility that dysbiosis, i.e., a change in the composition of the microbiota, could alter the immune system's response to certain allergens both locally and systemically. Accordingly, a link has been established between the gut microbiota and food allergies, the skin microbiome and atopic dermatitis, and the lung microbiota and respiratory allergies (13). On the other hand, patients with COVID-19, who had an abundance of opportunistic pathogens, exhibited an altered human microbiome in the stool and respiratory tract (14).
During the pandemic, both symptomatic and asymptomatic patients with COVID-19 overwhelmed hospitals. Because of their close interaction with the infectious virus, healthcare workers (HCWs) were on the front lines of treating and managing COVID-19 infections (15). HCWs were also a common and vulnerable group for these diseases due to the unpreparedness of the healthcare system and the unique nature of the SARS-CoV-2 infection. The spread of COVID-19 within hospitals became one of the most important routes of secondary transmission (16).
According to the literature, findings regarding the role of allergic diseases and asthma in the severity of COVID-19 infection are controversial (10, 17-19). Previous studies noted that 16% of patients with occupational asthma were HCWs, particularly hospital staff and nurses (20), who might be exposed to various allergens and triggers (21). On the other hand, because of their close contact with COVID-19 patients or their samples, HCWs are considered a high-risk group for developing COVID-19 (22, 23).
2. Objectives
This retrospective study aimed to clarify the clinical features and prognosis in allergic HCWs with confirmed COVID-19 infection.
3. Methods
3.1. Study Design and Participants
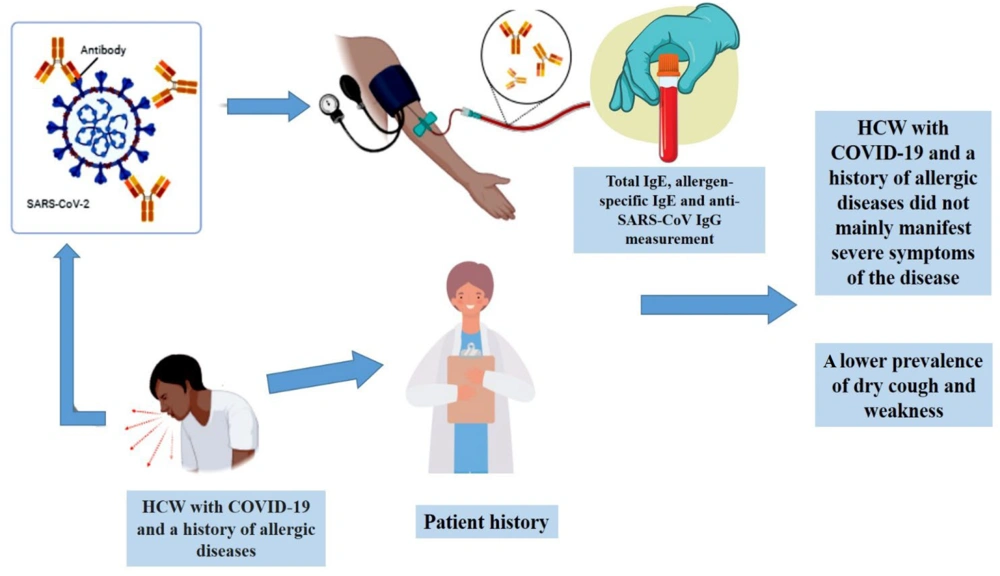
This retrospective study was conducted in Iran from February to June 2020. All patients were recruited from Kosar Hospital in Semnan, Iran. The study was approved by the Iran National Committee for Ethics in Biomedical Research (ID: IR.SEMUMS.REC.1399.033). A total of 30 HCWs with COVID-19 pneumonia were included as the study population, consisting of 15 non-allergic and 15 allergic HCWs. COVID-19 infection was confirmed through reverse-transcriptase polymerase chain reaction (RT-PCR) assays performed on nasopharyngeal swab specimens. For each patient, demographic data, clinical history, symptoms, and signs were documented. Patients with a history of allergic diseases and a positive allergen-specific immunoglobulin E (IgE) test were classified as allergic. Additionally, blood samples were collected from each participant, and sera were analyzed for antibody screening.
3.2. ELISA
To detect total IgE and anti-SARS-CoV IgG in the patients' serum, a commercial kit (Pishtazteb, Iran) was used following the manufacturer's instructions (24, 25).
3.3. Specific IgE Assay
The measurement of allergen-specific IgE was conducted using an immunoblot assay (Allesiascreen, MEDIWISS Analytic GmbH, Germany), designed to detect circulating specific sIgE against thirty food and aeroallergens in human serum (26). A specific IgE level > 0.35 IU/mL was considered positive.
3.4. Statistical Analysis
This study presented qualitative variables as counts and percentages using frequency distribution tables. Comparisons between the two groups (allergic and non-allergic individuals) were made using either the Pearson chi-Square test or Fisher's exact test. The normality of numerical variables was assessed with the Shapiro-Wilk test at a 99% confidence level. For numerical variables, the mean, standard deviation, median, and interquartile range (Q1 and Q3) were reported. Comparisons of numerical variables between the two groups were performed using the t-test and the Mann-Whitney U test at a 95% confidence level. SPSS version 16 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
4. Results
Females accounted for 73.3% of participants in the non-allergic group and 86.7% in the allergic group. The mean age was 34.1 years for non-allergic HCWs and 31.9 years for allergic HCWs. The demographics and basic characteristics of the patients are presented in Table 1. The results showed no significant differences in baseline characteristics between the two groups.
| Characteristics | Allergy | P-Value | |
|---|---|---|---|
| No | Yes | ||
| Age group (y) | 0.713 b | ||
| ≤ 30 | 6 (40.0) | 7 (46.7) | |
| > 30 | 9 (60.0) | 8 (53.3) | |
| Gender | 0.651 c | ||
| Female | 11 (73.3) | 13 (86.7) | |
| Male | 4 (26.7) | 2 (13.3) | |
| Job | > 0.9 c | ||
| Nurse | 12 (80.0) | 11 (73.3) | |
| Other | 3 (20.0) | 4 (26.7) | |
| Underlying diseases | > 0.9 c | ||
| No | 13 (86.7) | 14 (93.3) | |
| Yes | 2 (13.3) | 1 (6.7) | |
| Use of medications | > 0.9 c | ||
| No | 14 (93.3) | 13 (86.7) | |
| Yes | 1 (6.7) | 2 (13.3) | |
| Blood group | > 0.9 c | ||
| O | 6 (40.0) | 7 (46.7) | |
| A, B, AB | 9 (60.0) | 8 (53.3) | |
| Blood Rh | > 0.9 c | ||
| + | 14 (93.3) | 14 (93.3) | |
| - | 1 (6.7) | 1 (6.7) | |
a Values are expressed as No. (%).
b Pearson chi-square.
c Fisher's exact test.
The frequency distribution of participants in the two groups, based on their job roles, underlying diseases, medications used, blood groups, and types of IgE sensitization, is shown in Table 2. Most of the patients were nurses, suggesting a comparable level of exposure to workplace allergens. To reduce the confounding effects of underlying diseases, patients with conditions associated with a poor prognosis for COVID-19 infection were excluded from the study.
| Characteristics | Allergy | |
|---|---|---|
| No | Yes | |
| Job | ||
| Nurse | 12 (80.0) | 11 (73.3) |
| Doctor | 2 (13.3) | 2 (13.3) |
| Secretary | 0 (0.0) | 1 (6.7) |
| Practical nurse | 0 (0.0) | 1 (6.7) |
| Laboratory staff | 1 (6.7) | 0 (0.0) |
| Underlying diseases | ||
| Heart disease | 1 (6.7) | 0 (0.0) |
| Hypothyroidism | 0 (0.0) | 1 (6.7) |
| Migraine | 0 (0.0) | 1 (6.7) |
| Thyroid cancer | 1 (6.7) | 0 (0.0) |
| Nothing | 13 (86.7) | 13 (86.7) |
| Used medications | ||
| Levothyroxine | 0 (0.0) | 1 (6.7) |
| Metohexal + Aspirin | 1 (6.7) | 0 (0.0) |
| Relief | 0 (0.0) | 1 (6.7) |
| Nothing | 14 (93.3) | 13 (86.7) |
| Blood group | ||
| O+ | 5 (33.3) | 7 (46.7) |
| O- | 1 (6.7) | 0 (0.0) |
| A+ | 6 (40.0) | 4 (26.7) |
| B+ | 3 (20.0) | 1 (6.7) |
| B- | 0 (0.0) | 1 (6.7) |
| AB+ | 0 (0.0) | 2 (13.3) |
| Types of allergy | ||
| Respiratory | 0 (0) | 14 (93.3) |
| Food | 0 (0) | 3 (20) |
| Urticarial | 0 (0) | 2 (13.3) |
| Drug | 0 (0) | 2 (13.3) |
a Values are expressed as No. (%).
A comparison of clinical symptoms between the two groups revealed a significantly lower prevalence of dry cough in the allergic group (33.3%). Weakness was also observed less frequently in allergic patients (46.7% vs. 73.3%), although this difference did not reach statistical significance. No significant differences were identified in other symptoms between the allergic and control groups (see Table 3). Logistic regression analysis further indicated that the allergic group had a reduced risk of experiencing dry cough during the COVID-19 infection period.
| Clinical symptoms | Allergy | P-Value | ||
|---|---|---|---|---|
| All | No | Yes | ||
| Dyspnea | 0.715 a | |||
| No | 15 (50.0) | 8 (53.3) | 7 (46.7) | |
| Yes | 15 (50.0) | 7 (46.7) | 8 (53.3) | |
| Dry cough | 0.010 a | |||
| No | 13 (43.3) | 3 (20.0) | 10 (66.7) | |
| Yes | 17 (56.7) | 12 (80.0) | 5 (33.3) | |
| Fever | 0.456 a | |||
| No | 12 (40.0) | 5 (33.3) | 7 (46.7) | |
| Yes | 18 (60.0) | 10 (66.7) | 8 (53.3) | |
| Malaise | > 0.9 b | |||
| No | 7 (23.3) | 4 (26.7) | 3 (20.0) | |
| Yes | 23 (76.7) | 11 (73.3) | 12 (80.0) | |
| Anosmia | > 0.9 b | |||
| No | 23 (76.7) | 12 (80.0) | 11 (73.3) | |
| Yes | 7 (23.3) | 3 (20.0) | 4 (26.7) | |
| Loss of taste | 0.651 b | |||
| No | 24 (80.0) | 13 (86.7) | 11 (73.3) | |
| Yes | 6 (20.0) | 2 (13.3) | 4 (26.7) | |
| Diarrhea | 0.682 b | |||
| No | 22 (73.3) | 10 (66.7) | 12 (80.0) | |
| Yes | 8 (26.7) | 5 (33.3) | 3 (20.0) | |
| Nausea | 0.390 b | |||
| No | 23 (76.7) | 13 (86.7) | 10 (66.7) | |
| Yes | 7 (23.3) | 2 (13.3) | 5 (33.3) | |
| Weakness | 0.136 a | |||
| No | 12 (40.0) | 4 (26.7) | 8 (53.3) | |
| Yes | 18 (60.0) | 11 (73.3) | 7 (46.7) | |
| Chills | 0.464 a | |||
| No | 16 (53.3) | 7 (46.7) | 9 (60.0) | |
| Yes | 14 (46.7) | 8 (53.3) | 6 (40.0) | |
| Headache | 0.427 b | |||
| No | 21 (70.0) | 12 (80.0) | 9 (60.0) | |
| Yes | 9 (30.0) | 3 (20.0) | 6 (40.0) | |
| Sore throat | > 0.9 b | |||
| No | 26 (86.7) | 13 (86.7) | 13 (86.7) | |
| Yes | 4 (13.3) | 2 (13.3) | 2 (13.3) | |
| Anorexia | > 0.9 b | |||
| No | 21 (70.0) | 11 (73.3) | 10 (66.7) | |
| Yes | 9 (30.0) | 4 (26.7) | 5 (33.3) | |
a Pearson chi-square.
b Fisher's exact test.
According to Table 4, a significant difference was observed in the mean total IgE levels between the two groups (P = 0.004). However, no significant differences were found in age, disease duration, O2 saturation, or anti-SARS-CoV-2 IgG levels between the groups. The findings indicated that the duration of illness was shorter in patients with allergies (12.1 days compared to 15.6 days), although this difference was not statistically significant. Overall, these results suggest that the prognosis for COVID-19 patients with atopic diseases is not unfavorable.
| Variables | Allergy | P-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | ||||||||
| Mean ± SD | Median | Q1 | Q3 | Mean ± SD | Median | Q1 | Q3 | ||
| Age | 34.1 ± 7.2 | 33.0 | 26.0 | 41.0 | 31.9 ± 6.6 | 31.0 | 27.0 | 39.0 | 0.404 a |
| Duration of disease (d) | 15.6 ± 8.9 | 14.0 | 8.0 | 21.0 | 12.1 ± 5.5 | 11.0 | 7.0 | 18.0 | 0.202 a |
| O2 saturation | 93.7 ± 1.9 | 93.0 | 92.0 | 95.0 | 92.8 ± 4.2 | 95.0 | 92.0 | 95.0 | 0.967 b |
| IgG | 11.91 ± 5.65 | 11.95 | 7.30 | 17.61 | 10.58 ± 5.59 | 8.74 | 6.32 | 15.44 | 0.521 a |
| IgE | 96.67 ± 93.11 | 68.25 | 37.81 | 127.51 | 205.04 ± 131.58 | 188.38 | 76.34 | 334.98 | 0.004 b |
at-test.
b Mann-Whitney U.
5. Discussion
This study investigates the various clinical manifestations of COVID-19 in patients with allergic diseases in Semnan, Iran. The findings showed no significant differences in age, disease duration, oxygen saturation, or anti-SARS-CoV-2 IgG levels between the two groups. However, a significantly lower percentage of dry cough was observed in allergic patients with COVID-19 compared to those without allergic conditions.
Although some previous studies have suggested that respiratory allergies, asthma, and obesity are risk factors for acute respiratory distress syndrome (ARDS) in patients with COVID-19 (27), few have documented severe COVID-19 in patients with allergies (12, 28). Moreover, a cohort study by Yang et al., which included 219,959 adults, demonstrated a positive association between asthma—primarily non-allergic types—and acute respiratory distress, as indicated by SARS-CoV-2 test positivity and the severity of COVID-19 infection (18). Yang et al. identified several potential reasons for this association, including the large sample size, upregulation of transmembrane protease serine 2 (TMPRSS2) in airway cells, induction of local inflammatory processes by respiratory viruses, subsequent recruitment of immune cells, cytokine production, activation of TH2 pathways, and increased mucin secretion (18).
In contrast to Yang et al.'s findings, a meta-analysis conducted by Wu et al. revealed a very low likelihood of asthma in patients with severe COVID-19 who were hospitalized or died (29). Furthermore, Garg et al. reported that asthma was an underlying condition in 17% of inpatients with COVID-19 across 14 US states, particularly among those aged 18 to 49 years (27.3%) (4).
The results of Dong et al.'s study on 11 patients with COVID-19 in Wuhan, China, revealed that severe symptoms and manifestations of COVID-19 were not observed in three patients with AR, atopic dermatitis, or urticaria (19). Similarly, Zhang et al., in a study involving 140 COVID-19 patients in Wuhan, reported that 18 individuals (12%) had a history of allergic diseases, including 16 cases of drug allergies and 2 cases of chronic urticaria. Of these 18 patients, seven experienced severe forms of COVID-19. This study concluded that an allergic background cannot be considered a primary predictor of SARS-CoV-2 infection (17).
In another study by Garcia-Menaya et al., out of 113 patients with COVID-19, 24 had a history of allergic diseases, including 11 with drug hypersensitivity, 7 with asthma, and 8 with AR. The study found no significant differences in laboratory and radiological findings between COVID-19 patients with and without allergies (30). Furthermore, considering the antiviral effects of eosinophils (31), studies have shown that patients with the TH2 phenotype of asthma and eosinophilia have a better prognosis for COVID-19 compared to those with other phenotypes (32).
According to the results of this study, HCW with COVID-19 infection and a history of allergic symptoms had a lower risk of developing a dry cough compared to workers without allergic symptoms. Similarly, in Garcia-Menaya study, the manifestations of COVID-19 infection were compared between patients with and without allergic diseases, and no significant differences were observed between the two groups. In contrast to our findings, Garcia-Menaya’s study found no difference in the incidence of dry cough. However, both studies observed that asthenia (weakness) occurred in a lower percentage of allergic patients (30).
Various hypotheses may explain the lower severity of COVID-19 in patients with allergic diseases. One potential explanation is the downregulation of ACE2 expression in airway cells in individuals with allergic asthma and AR compared to those with non-allergic asthma (33, 34). Other factors that may contribute include reduced levels of interferons and the use of inhaled corticosteroids and omalizumab (35). Additionally, eosinophils, which play a central role in allergic inflammation (36), may offer protective effects. Studies have demonstrated that the lungs of allergic mice are better protected against the influenza virus, possibly due to eosinophil-induced cellular immunity (37). Similarly, in a study conducted by Chen et al., a positive correlation was observed between higher eosinophil counts and a better prognosis in COVID-19 patients. Moreover, lower mortality rates were reported in patients with asthma (1%) and allergic diseases (2.9%) (38).
In this study, the duration of illness in COVID-19 patients with allergic diseases was shorter than in non-allergic individuals, although the difference was not statistically significant. This finding aligns with Shi et al.'s research, which reported a lower prevalence of severe COVID-19 in allergic patients (57.2% vs. 84.1%) and a shorter time to nucleic acid negativity in allergic patients (39).
The objective of this retrospective study was to investigate the clinical characteristics and prognosis of allergic HCWs with confirmed COVID-19 infection. The results suggest that HCWs with COVID-19 and a history of allergic disease did not predominantly experience severe symptoms. In particular, allergic patients reported a lower prevalence of dry cough and weakness (Figure 1). However, two limitations of this study should be acknowledged: The relatively small sample size and the lack of data on eosinophil counts for each patient. Addressing these limitations in future research could provide a more comprehensive understanding of the relationship between allergies and COVID-19 severity.