1. Background
Severe acute respiratory infections (SARI) have long posed a significant threat to global public health, causing substantial illness and death across all age groups (1). Viral infections are the primary causes of mortality from SARI, with influenza, respiratory syncytial virus, rhinovirus, and human metapneumovirus being the main pathogens involved (2-4). Annually, SARI accounts for approximately 4.2 million deaths worldwide, with children under the age of five being particularly at risk, representing 1.4 million of these fatalities (5-7). Influenza alone affects 5 - 15% of the population and is responsible for an estimated 291,000 to 650,000 deaths globally each year (8).
The outbreak of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), significantly exacerbated the challenges associated with SARI, leading to approximately 6.9 million deaths globally (1). During this period, there was a marked increase in SARI cases linked to SARS-CoV-2, resulting in higher rates of in-hospital fatalities and 30-day mortality following discharge (9). The pandemic underscored that SARI-related deaths were no longer attributed solely to influenza but were profoundly influenced by COVID-19. Countries such as Brazil and India experienced a significant surge in both SARI cases and deaths during the pandemic (10, 11).
Understanding the factors contributing to COVID-19 mortality is crucial for improving patient outcomes and informing public health responses. Among the various therapeutic options explored, repurposed antiviral drugs have been at the forefront of treatment strategies for COVID- 19 (12, 13). Notably, remdesivir (RDV) has shown promise in reducing recovery time in hospitalized patients with severe disease. It acts as a nucleotide analog that inhibits viral RNA synthesis, thereby limiting viral replication. Clinical trials have demonstrated that patients treated with RDV recover approximately five days faster than those receiving placebo treatment (14).
Oseltamivir (OST), primarily used for influenza, has also been investigated for its potential benefits in COVID-19 management, although its efficacy remains debated. Some studies suggest it may help reduce viral load and improve clinical outcomes, particularly in patients presenting with respiratory symptoms (15). Lopinavir/ritonavir (LPV/r), an antiviral combination initially developed for HIV treatment, has been evaluated in several trials for COVID-19. While some studies indicated a reduction in viral load, results regarding its effectiveness in improving clinical outcomes have been mixed (16).
This study aims to analyze antiviral drug usage among patients who died from COVID-19 at Razi Hospital between 2019 and 2022.
2. Objectives
The objectives of this study were to investigate the association of repurposed antiviral drugs with COVID-19 mortality.
3. Methods
3.1. Design and Settings
This study employed an analytical cross-sectional design to evaluate the clinical and demographic characteristics of patients who died from COVID-19 at Razi Hospital in Rasht between 2019 and 2022. A census sampling method was utilized, including all eligible cases of COVID-19 deaths recorded during the specified period. Patients were included if their records contained complete information on key variables, while those with missing or incomplete data were excluded.
3.2. Data Collection
Data were retrospectively collected from patient files, including details such as age, sex, and antiviral medication usage. The data were coded and entered into SPSS version 16 for analysis. Descriptive statistics were employed to summarize continuous variables (e.g., age, oxygen saturation, number of comorbidities, number of drugs used) and categorical variables (e.g., sex, insurance status, ICU admission, comorbidities, antiviral drug type). Means, standard deviations, medians, interquartile ranges, and frequency percentages were calculated for the relevant variables.
3.3. Ethical Considerations
The research was approved by the Ethics Committee of Guilan University of Medical Sciences (IR.GUMS.REC.1401.272) in Guilan, Iran.
3.4. Statistical Analysis
For inferential analysis, the Mann-Whitney U test was employed to compare continuous variables such as age and oxygen saturation between patients who received antiviral medications and those who did not. Chi-square tests were conducted to evaluate associations between clinical binary variables (e.g., sex, ICU admission, underlying conditions) and antiviral drug use. Additionally, the Cochran-Armitage trend test was used to examine the relationship between the number of underlying conditions and the frequency of antiviral drug use. A P-value of less than 0.05 was considered statistically significant for all analyses.
4. Results
The demographic and clinical characteristics of patients who died from COVID-19 at Razi Hospital between 2019 and 2022 are presented in Table 1.
| Variables | Mean ± SD or No. (%) |
|---|---|
| Age (y) | 64.8 ± 14.8 |
| Gender | |
| Male | 764 (51.7) |
| Female | 713 (48.3) |
| Insurance status | |
| Insured | 1341 (90.8) |
| Not insured | 136 (9.2) |
| O2 sat | 86.7 (2.52) |
| ICU admission | |
| Yes | 469 ± 31.8 |
| No | 1008 ± 68.2 |
| Comorbidities | |
| Hypertension | 578 (39.1) |
| Diabetes | 524 (35.5) |
| Cardiovascular disease | 492 (33.3) |
| Pulmonary disease | 459 (31.1) |
| Renal disease | 437 (29.6) |
| Number of comorbidities | |
| 0 | 252 (17.1) |
| 1 | 389 (26.3) |
| 2 | 421 (28.5) |
| ≥ 3 | 415 (28.1) |
| Antiviral drug type | |
| RDV | 664 (45.0) |
| IFN-β-1a | 388 (26.3) |
| OST | 186 (12.6) |
| LPV/r | 169 (11.4) |
| Number of drugs used | |
| 0 | 475 (32.2) |
| 1 | 609 (41.2) |
| 2 | 381 (25.8) |
| 3 | 12 (0.8) |
Abbreviations: RDV, remdesivir; OST, oseltamivir; LPV/r, lopinavir/ritonavir; O2 sat, oxygen saturation.
The results indicated that RDV was administered more frequently to females (47.7%) than males (42.8%), although this difference was not statistically significant (P = 0.085). In contrast, OST and LPV/r were more commonly used in males (15.4% and 13.9%, respectively). Statistically significant differences were observed in the use of OST (P = 0.001) and LPV/r (P = 0.002) between genders. Additionally, insured patients were significantly more likely to receive RDV (P = 0.006) and interferon beta-1a (INF-β-1a) (P = 0.009) compared to uninsured patients (Table 2).
| Variables and Categories | RDV | INF-β-1a | OST | LPV/r |
|---|---|---|---|---|
| Gender | ||||
| Male (n = 764) | 42.8 | 24.2 | 15.4 | 13.9 |
| Female (n = 713) | 47.7 | 28.5 | 9.5 | 8.8 |
| P-value | 0.085 | 0.063 | 0.001 | 0.002 |
| Insurance | ||||
| Yes (n = 1341) | 46.1 | 27.1 | 11.6 | 11.2 |
| No (n = 136) | 33.8 | 16.9 | 22.1 | 14.0 |
| P-value | 0.006 | 0.009 | < 0.001 | 0.331 |
| ICU admission | ||||
| Yes (n = 469) | 63.8 | 34.1 | 8.1 | 9.0 |
| No (n = 1008) | 36.2 | 22.6 | 14.7 | 12.6 |
| P-value | < 0.001 | < 0.001 | < 0.001 | 0.041 |
| Comorbidities | ||||
| Yes (n = 1225) | 47.2 | 27.2 | 11.4 | 11.2 |
| No (n = 252) | 34.1 | 21.8 | 18.3 | 12.7 |
| P-value | < 0.001 | 0.078 | 0.003 | 0.491 |
| Number of comorbidities | ||||
| 0 (n = 252) | 34.1 | 21.8 | 18.3 | 12.7 |
| 1 (n = 609) | 40.9 | 26.5 | 14.4 | 13.1 |
| 2 (n = 381) | 25.8 | 14.6 | 11.2 | 9.4 |
| ≥ 3 (n = 475) | 53.5 | 28.4 | 8.4 | 9.4 |
| P-value | < 0.001 | 0.088 | 0.002 | 0.100 |
| Hypertension | ||||
| Yes (n = 578) | 50.9 | 26.6 | 8.3 | 9.2 |
| No (n = 899) | 46.1 | 26.0 | 15.4 | 12.9 |
| P-value | < 0.001 | 0.793 | < 0.001 | 0.028 |
| Diabetes | ||||
| Yes (n = 492) | 50.6 | 27.0 | 9.1 | 10.2 |
| No (n = 985) | 42.1 | 25.9 | 14.3 | 12.1 |
| P-value | 0.002 | 0.638 | 0.005 | 0.275 |
Abbreviations: RDV, remdesivir; INF-β-1a, interferon beta-1a; OST, oseltamivir; LPV/r, lopinavir/ritonavir.
a Values are expressed as %.
ICU admission was strongly associated with increased antiviral drug use. Table 2 shows that ICU patients had significantly higher usage rates of RDV (63.8%), INF-β-1a (34.1%), and OST (8.1%) compared to non-ICU patients. These differences were highly statistically significant for all three drugs (P < 0.001). Similarly, patients with multiple comorbidities were more likely to receive RDV and OST, with the highest usage rates observed among those with three or more comorbidities (53.5% for RDV and 18.3% for OST) (P < 0.001 and P = 0.002, respectively) (Table 2).
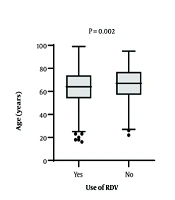
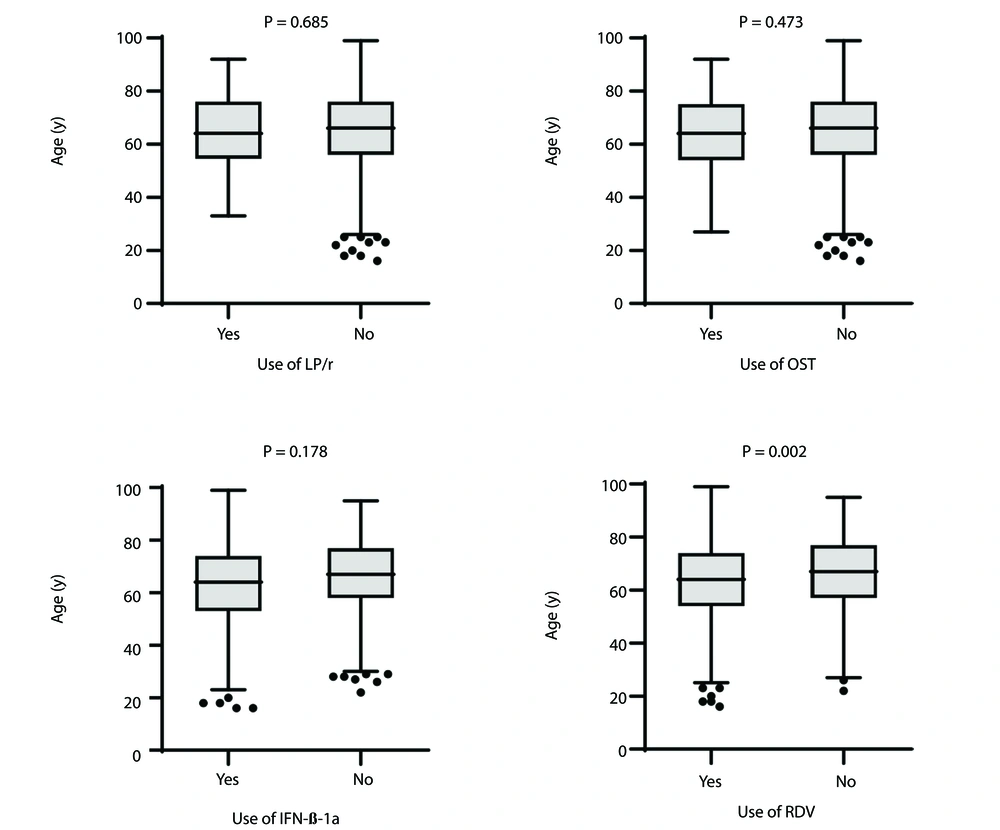
Figures 1 and 2 explore the relationship between antiviral drug use, patient age, and oxygen saturation (O2 sat). There were no significant differences in age between patients who received INF-β-1a, OST, or LPV/r and those who did not (P = 0.178, 0.473, and 0.685, respectively).
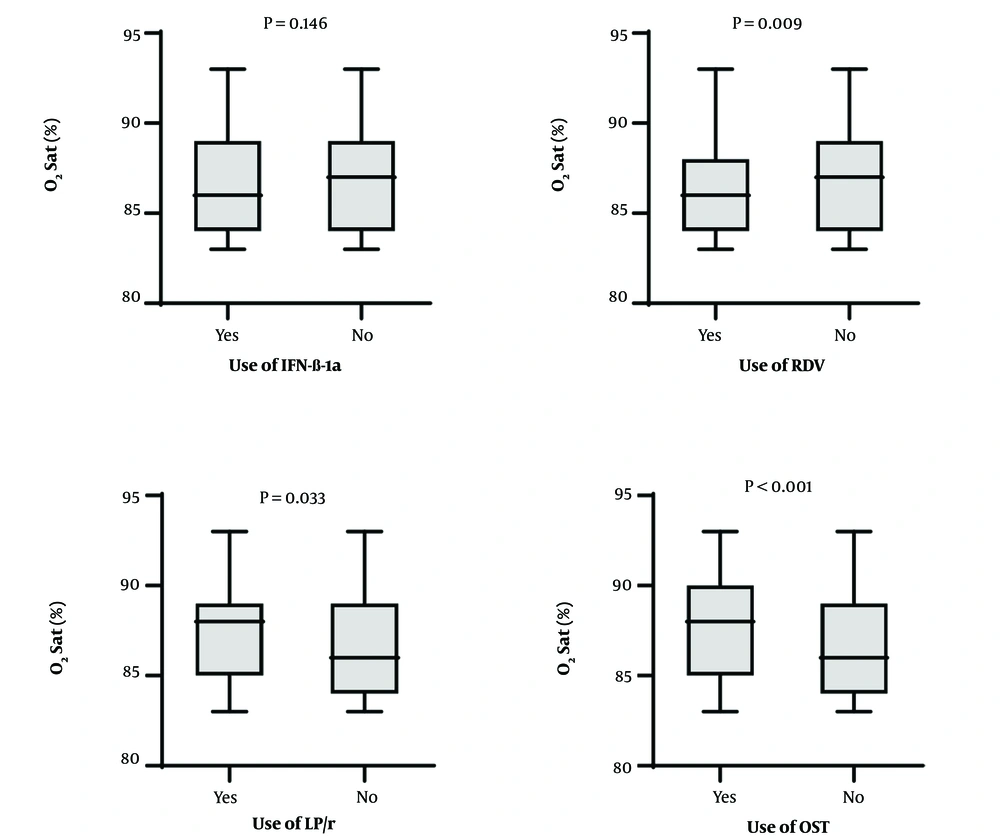
Oxygen saturation levels at the time of admission were significantly lower among patients who received RDV, OST, and LPV/r compared to those who did not receive these medications (P = 0.006, P < 0.001, and P = 0.033, respectively). This suggests that these drugs were more commonly administered to patients experiencing more severe respiratory distress (Figure 2). However, no significant differences in oxygen saturation levels were found between patients who did and did not receive INF-β-1a (P = 0.146).
5. Discussion
This study analyzed antiviral drug usage among 1,477 patients who died from COVID-19 at Razi Hospital, providing critical insights into treatment patterns and their association with mortality. Notably, a significant proportion of these patients (32.2%) did not receive any antiviral treatment, while among those who did, RDV was the most commonly administered drug (45%). This finding aligns with previous research indicating that RDV is frequently prioritized in clinical settings due to its demonstrated efficacy in reducing recovery time for hospitalized COVID-19 patients (17-19). A randomized trial demonstrated that patients treated with RDV recovered five days faster on average than those given a placebo (20). Additionally, RDV has shown inhibitory effects on coronaviruses such as SARS-CoV and MERS-CoV in vitro, outperforming lopinavir and ritonavir in laboratory and animal models (21, 22).
Interestingly, RDV was prescribed more frequently for younger patients with more severe conditions, lower oxygen saturation levels, and those admitted to the ICU, indicating its selective use based on clinical need. This suggests that it was more commonly administered to patients with a higher potential for survival rather than being used routinely for all cases.
A study by Lai et al. reported that RDV treatment reduced mortality rates among hospitalized patients, supporting our observation that it is frequently used for patients with more severe conditions (22). Furthermore, Beigel et al. found that patients treated with RDV experienced an average recovery time that was five days shorter than those receiving placebo, reinforcing the idea that timely administration of this antiviral can significantly improve patient outcomes (23). In contrast, our study observed that LPV/r was used in only 11.4% of patients, consistent with findings from Cao and Li, who reported mixed results regarding its effectiveness in treating COVID-19 (24). This indicates a shift in clinical practice as more evidence emerges regarding the limited benefits of LPV/r compared to other antiviral agents like RDV.
Interferon beta-1a was administered to 388 (26.3%) patients, but its effectiveness remains questionable. For example, a study involving a 50-year-old patient reported no improvement in symptoms when Interferon was used alongside LPV/r (25). This aligns with the findings in this study, where these treatments were more frequently administered to critically ill, insured patients, likely influenced by the high cost of these drugs.
Lopinavir/ritonavir was used in 169 (11.4%) patients, though its effectiveness against COVID-19 remains a topic of debate. Some studies suggest it may help reduce the viral load, but conclusive evidence on its clinical benefits is lacking (26). In one instance, it was observed to lower the viral load after a single day of treatment, although clinical outcomes were mixed (27).
Oseltamivir was administered to 186 (12.6%) patients. Other researchs associated OST with reduced mortality and shorter hospital stays (28, 29). In this study, patients who received OST had higher oxygen saturation levels, suggesting that it was more often prescribed to patients in relatively better clinical condition.
Overall, the use of antiviral drugs varied significantly, with prescribing decisions influenced by factors such as patient condition, age, and access to healthcare resources like insurance. Remdesivir and other antivirals were not universally administered but were more commonly prescribed to younger, critically ill patients, reflecting a selective approach to their use.
5.1. Limitations
One notable limitation of this research is that it was conducted in a university-affiliated medical center, which restricts the generalizability of the results. Additionally, the study's cross-sectional design makes it challenging to establish definitive causal relationships. The absence of confounder-adjusted estimates and confidence intervals in the results raises concerns about potential bias, generalizability, causal inference, and precision. Addressing these limitations in future research will enhance the validity and applicability of findings related to the effectiveness of training in ethical awareness among health professionals.
Future research should include larger, multicenter, prospective studies to provide more robust evidence. Systematic reviews and meta-analyses are also recommended to consolidate findings from various studies on this topic. A practical recommendation from this study is the development of national guidelines for the use of antiviral medications in treating COVID-19 patients, customized to align with the clinical condition of each patient.
5.2. Conclusions
The findings of this study indicate that approximately one-third of COVID-19-related deaths occurred in patients who had not received any antiviral treatment. Among those who did receive treatment, the majority were administered only one antiviral drug, with RDV being the most commonly used.