1. Context
The gut microbiota (GM) comprises a diverse array of microorganisms residing in the gastrointestinal tract, forming a complex ecosystem that includes both identified and unidentified species of bacteria, archaea, fungi, protists, and viruses (1). The estimated population of microorganisms in the gastrointestinal tract is believed to surpass 1014, with a weight of approximately 2 kg, representing about ten times the number of bacterial cells compared to human cells and more than one hundred times the genomic content (microbiome) relative to the human genome (2). These microorganisms reside within the human intestines and are crucial for maintaining human health (3). This microbiological population undergoes renewal every three days (4). The GM plays a crucial role in digestion, immune defense, and the regulation of the nervous system, particularly in relation to metabolism, while sustaining a delicate equilibrium with the human host (5). This bio-diverse microbial community has evolved over millions of years, resulting in a mutualistic relationship that impacts essential human life processes, ranging from digestion to systemic maintenance (6). Recent scientific advancements have highlighted the significant influence of the microbiome on various aspects of human health, including genetic expression, immune function, metabolic balance, mental well-being, regulation of gastrointestinal hormone secretion, and the reduction of risk factors associated with numerous diseases (7). It is important to recognize that the composition of GM varies significantly among individuals, and a strategy that proves effective for one person may not yield the same results for another (8). The GM can be regarded as a dynamic environmental element, as its composition is influenced by host genetics, dietary habits, hygiene practices, and lifestyle choices. This variability positions it as a potential therapeutic target for cardiovascular disease (CVD) and myocardial infarction (MI) (9). The presence of varying bacterial composition and diversity within atherosclerotic plaques, alongside the systemic circulation of patients with CVDs, suggests a correlation between alterations in GM composition and the occurrence of CVD (10).
2. Evidence Acquisition
2.1. Overview of Gut Microbiota
The recent surge in scientific research highlights the significant influence of the microbiome on various aspects of human health, including genetic expression, immune function, metabolic balance, mental well-being, regulation of gastrointestinal hormone secretion, and the reduction of risk factors associated with a range of diseases (7). The GM, consisting of trillions of microorganisms residing in the gastrointestinal tract, can be regarded as a sophisticated bioreactor that exerts various metabolic and immunological influences, which reach beyond the confines of the gut. Over the past ten years, a growing body of evidence has established connections between the functions and changes in the GM and the development of cardio-metabolic diseases (11). The GM has recently been regarded as an essential "invisible organ" (12).
2.2. The Function of the Gut Microbiome
Alterations in the intestinal microbiota may facilitate the onset of intricate metabolic disorders, including obesity and atherosclerosis (13). Animal heart models illustrate the protective benefits of symbiotic microbiota in cases of ischemic heart conditions, hypertension, and MI (14). The presence of a healthy GM can regulate immune responses and diminish the size of MI as well as the severity of heart failure (HF) following an MI (15). The specific ways in which a beneficial microbiome may lower the risk factors associated with MI or diminish post-MI occurrences involve the regulation of lipogenesis and cholesterol metabolism, as well as the generation of antioxidants. Additionally, it can improve the host's immune and cellular responses, while also influencing gene expression and physiological processes within the host (9). Beneficial microbiota reduce leptin concentrations (16). Under pathological conditions such as obesity, the effects of leptin may alter, leading to the induction of oxidative stress, inflammation within immune cells, and hypertrophy of vascular smooth muscle. Additionally, it enhances the production of reactive oxygen species (ROS) in endothelial cells (17). An increased level of leptin is suggested as an additional risk factor for the progression of atherosclerosis (18). Balanced microbiota influences cholesterol metabolism. Balanced microbiota can stimulate liver X receptor (LXR) in macrophages, which in turn enhances the expression of genes related to the absorption, transport, efflux, and elimination of cholesterol (19). Beneficial bacteria utilize intestinal fiber to synthesize acids, such as propionic acid, which reduce cholesterol synthesis in the liver. These bacteria can also break down bile acids (BAs) generated from cholesterol, facilitating the digestion of fats in the body. As a result, they stimulate the liver to generate more BAs from cholesterol, using cholesterol as a source of nourishment (20). Microbiota-derived short-chain fatty acids (SCFAs) play a significant role in the regulation and development of blood pressure (21).
2.3. Gut Dysbiosis
The disruption or maladjustment of the microbial community, referred to as "dysbiosis", has been linked to the occurrence of CVD risk and influences the advancement of CVD. Notable dysbiosis has been observed in hypertensive animals, characterized by a reduction in microbial richness and diversity, along with an elevated Firmicutes/Bacteroidetes (F/B) ratio (22). The configuration and functioning of the GM, which can be influenced by human genetic and environmental factors such as infections, dietary habits, or lifestyle choices, may disrupt this symbiotic relationship and affect the biology of the host in various manners (5). Numerous factors, such as stress, dietary habits, increased levels of inflammatory markers, and the use of antibiotics, contribute to alterations in the composition of GM, a condition referred to as gut dysbiosis (23). Dysbiosis plays a significant role in the onset and advancement of atherosclerosis and CVD by contributing to dyslipidemia, inflammation, and arterial fibrosis (24).
3. The Influence of Dysbiosis in Gut Microbiota on Cardiovascular Disease
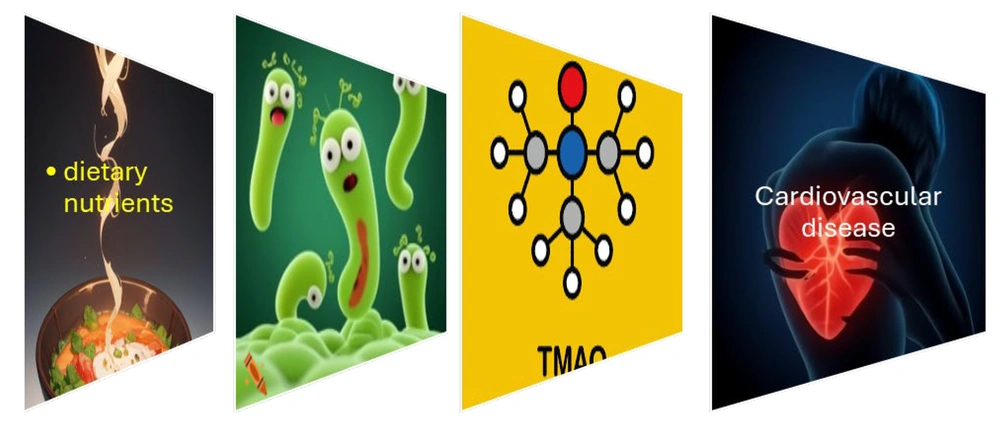
Environmental factors, including obesity, lifestyle choices, and dietary habits, contribute to microbial dysbiosis and the proliferation of pathogens, which ultimately result in the compromise of barrier integrity and the disruption of the epithelial barrier (25). The movement of bacteria and their constituents into the systemic circulation via the permeable gut initiates systemic endotoxemia and inflammatory responses (26). The activation of the nuclear factor kappa B (NF-kB) pathway in macrophages and epithelial cells results in the synthesis of pro-inflammatory cytokines. Dietary phosphatidylcholine is metabolized by the GM into trimethylamine (TMA), which readily enters the bloodstream and is subsequently oxidized by the liver to produce trimethylamine N-oxide (TMAO), a compound linked to CVDs in the host (27).
4. Alteration of the Gut Microbiome in Patients with Heart Failure
Alterations in the levels of metabolites such as SCFAs, TMAO, various amino acid metabolites, and different BA species have been demonstrated to lead to gut dysbiosis and the exacerbation of HF (28). The microbiome can delay or block the development of post-infarction HF.
5. Thrombosis
Changes in microbiota were identified in Ldlr−/− mice, which serve as a model for hypercholesterolemic atherosclerosis, accompanied by an increased rate of arterial thrombosis (29). Changes in various bacterial groups were also observed in cohorts with CVD, including a reduced proportion of Bacteroides and Roseburia (30). Trimethylamine N-oxide engages with platelets, leading to heightened platelet reactivity and consequently elevating the risk of thrombosis (31).
6. Hyperlipidemia
Intestinal microbes affect CVD through the modulation of the host's lipid metabolism. The GM plays a significant role in lipid metabolism disorders, including dyslipidemia and hyperlipidemia, which are key contributors to the risk of developing CVD (32). Changes in microbiota have been linked to dysfunctions in lipid metabolism, such as reduced levels of high-density lipoprotein (HDL) in the plasma of patients with CVD (33).
7. Hypertension and Gut Microbiota
The GM significantly influences blood pressure management (34). Antibiotic treatment, which reduces GM, has been shown to increase blood pressure in rats (35). Hypertensive animals exhibit a notable decrease in microbial richness and diversity, along with an increased F/B ratio (22). In individuals with hypertension, changes in GM are characterized by reduced α-diversity and a notable alteration in β-diversity. This is marked by an increased presence of potentially pathogenic bacteria such as Parabacteroides, Desulfovibrio, Prevotella, and Oscillibacter. These gram-negative bacteria can produce endotoxins, including lipopolysaccharides (LPS), which are linked to inflammatory conditions. Additionally, certain gram-positive bacteria, particularly Clostridium, have been observed in greater quantities among hypertensive patients (36).
8. Atherosclerosis and Microbiota
Microbiota plays a significant role in the development of atherosclerosis through various mechanisms. Dysbiosis in the gut leads to increased permeability, allowing bacteria and their metabolites, such as LPS, to enter the bloodstream. The heightened presence of circulating LPS contributes to inflammation and the formation of foam cells through several pathways. Foam cell formation begins when apolipoprotein B, found on the surface of circulating low-density lipoproteins (LDLs), binds to LDL receptors on endothelial cells, resulting in the endocytosis of LDL into the tunica intima. This process leads to the oxidation of LDL, producing oxidized low-density lipoproteins (oxLDLs). These oxLDLs promote the differentiation of monocytes into macrophages by enhancing the production of macrophages colony-stimulating factor (M-CSF). Additionally, the accumulation of oxLDLs in the arterial wall stimulates the expression of cell adhesion molecules on endothelial cells. Moreover, oxLDLs are internalized by scavenger receptors (ScRs) on macrophages, while LPS interacts with Toll-like receptor 4 (TLR4), triggering the release of pro-inflammatory cytokines (37).
The gut microbiome, particularly the bacterial genera Prevotella, Bacteroides, Clostridium, and Faecalibacterium, plays a significant role in the development of atherosclerosis through its involvement in lipid metabolism (38). A comprehensive study involving a substantial cohort of patients with atherosclerotic cardiovascular disease (ACVD) revealed a significant disruption in the composition of the gut microbiome. The analysis indicated an increased prevalence of Enterobacteriaceae, including Escherichia coli, Enterobacter aerogenes, and Klebsiella species, alongside elevated levels of Streptococcus species and Eggerthella lenta, which possesses enzymes responsible for the deactivation of cardiac medications. In contrast, butyrate-producing bacteria, such as Roseburia intestinalis and Faecalibacterium cf. prausnitzii, as well as other typical constituents of the gut microbiome, were found to be diminished in patients with ACVD when compared to healthy individuals (39).
9. Microbiota and Atherosclerosis Development
The absence of microbiota in the ApoE−/− mouse model on a standard diet expedited the development of atherosclerotic plaques in the aorta and the onset of heart diseases, in contrast to conventional mice (40). Endotoxin, upon entering the bloodstream, has the potential to damage endothelial cells by interacting with TLR-4 present on the cell surface. This interaction promotes the production of ROS, which in turn diminishes the bioavailability of nitric oxide (NO) in endothelial cells. Consequently, this process contributes to plaque development and the progression of atherosclerotic lesions (41). This hypothesis has been validated through studies involving animal models, specifically ApoE−/− mice subjected to a western diet, which exhibited an exacerbation of atherosclerotic lesions alongside a notable rise in Proteobacteria (gram-negative pro-inflammatory bacteria) and systemic levels of LPS (42). The lack of GM appears to reduce the atherogenic impact associated with prolonged consumption of dietary lipids (29).
The presence of microbiota is essential for the production of TMAO, which plays a role in the progression of atherosclerosis through various mechanisms. One such mechanism involves the formation of foam cells. Trimethylamine N-oxide derived from microbiota can induce the expression of stress-related heat-shock proteins, specifically HSP70 and HSP60. This induction may lead to the activation of scavenger receptors, such as SR-A1 and CD36, in macrophages, thereby promoting the uptake of oxLDLs and facilitating the formation of foam cells (43).
10. Obesity and Type 2 Diabetes Mellitus
The GM plays a crucial role as an environmental factor influencing the regulation of body weight and energy balance. Consequently, it is reasonable to assert that microbiota is associated with type 2 diabetes mellitus, insulin resistance, and obesity, which are recognized as traditional risk factors for CVD (44). A connection exists among dietary consumption, GM, and liver metabolism (45). Dysbiosis in GM can affect the efficiency of energy extraction from dietary sources, thereby impacting the likelihood of developing obesity and atherosclerosis through the regulation of inflammation and lipid metabolism (46).
11. Antioxidant Properties of Symbiotic Microbiome
The process by which synbiotics exert their antioxidant properties has been associated with their capacity to activate and relocate nuclear factors. These factors stimulate the expression of the enzymatic system responsible for antioxidant defense, generate essential antioxidant molecules, and facilitate the detoxification of singlet oxygen and free radicals. Lactobacillus and Bifidobacterium strains are considered the most important probiotics that contribute to synbiotic antioxidant activities. Research has indicated that synbiotics, which consist of Lactobacillus casei and inulin, are effective agents in safeguarding the human body against damage inflicted by free radicals. These synbiotics have the potential to enhance the antioxidant capacity of blood plasma as well as the functionality of specific antioxidant enzymes (47).
12. Dietary Factors and Gut Microbiota
A connection has been established between specific gut microbes, dietary nutrients, platelet function, and the risk of thrombosis. Research conducted in both animals and humans has examined the prothrombotic effects associated with dietary choline and elevated levels of the gut microbe-dependent metabolite TMAO (48). A diet characterized by plant-based (high-fiber) and animal-based (high-fat) components resulted in a notable alteration in the microbiota composition of all participants within a span of 1 to 2 days. This change was marked by a heightened presence of Firmicutes, which are responsible for metabolizing dietary fiber in the context of a plant-based diet, alongside an increase in bile-tolerant microorganisms such as Alistipes and Bilophila associated with an animal-based diet (49).
Long-term dietary interventions are linked not only to changes in composition but also to physiological modifications. For example, administering a high-fat diet (HFD) to rats for a duration of 8 or 12 weeks resulted in a heightened presence of Enterobacteriales (within the Proteobacteria phylum), which was associated with an increase in systemic inflammation, intestinal permeability, and the development of an obese phenotype (49). Dietary modifications can lead to distinct alterations in microbial composition. For instance, an increase in dietary fiber consumption has been associated with enhanced richness and diversity of GM, particularly within the Firmicutes phylum (50).
A diet high in red meat resulted in elevated levels of TMAO, an intestinal microbial metabolite derived from choline found in red meat, in the plasma of individuals compared to vegetarians. Increased TMAO levels have been observed in human subjects with a higher prevalence of the enterotype Prevotella, which is linked to a greater risk of CVD (51). Dietary fats have been recognized as factors that compromise the intestinal barrier by stimulating the release of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFNγ), and interleukin-1 beta (IL-1β) (52). The increased expression of pro-inflammatory cytokines subsequently stimulated the myosin light-chain kinase (MLCK) signaling pathway, leading to the reorganization of tight junction proteins such as occludin and zonula occludens-1 (ZO-1), ultimately contributing to the development of a leaky gut (53).
When the integrity of the intestinal barrier is compromised, LPS or pathogens may enter the bloodstream, leading to endotoxemia, which in turn triggers the release of systemic pro-inflammatory cytokines (54). A diet high in lipids also exacerbated microbiota dysbiosis in Ldlr−/− mice, resulting in an increased prevalence of Clostridiaceae, Staphylococcaceae, and Bacillales, while the abundance of Lactobacillaceae was reduced (29). Dietary metabolites of phosphatidylcholine, such as choline, TMAO, and betaine, play significant roles as risk factors for CVD (55). In the gastrointestinal tract, L-carnitine or phosphatidylcholine, which is the primary dietary source of choline, can be metabolized into TMA by the microbiota (56). Trimethylamine N-oxide influences the metabolic processes of cholesterol and sterol within macrophages, the liver, and the intestines (57).
13. Trimethylamine and Trimethylamine-N-oxide
Trimethylamine-N-oxide, a co-metabolite produced by GM from dietary nutrients, was initially identified and documented as a predictor of CVD risk approximately ten years ago (Figure 1) (58). The dietary components phosphatidylcholine, choline, and L-carnitine, frequently found in cheese, red meat, seafood, egg yolks, and other Western dietary sources, are predominantly processed by particular enzymes produced by GM, resulting in elevated concentrations of TMA (59). Trimethylamine is subsequently taken up into the bloodstream and metabolized in the liver by flavin monooxygenases (FMOs, predominantly FMO3), resulting in the production of TMAO (60). Seven distinct bacterial strains that express the TMA lyase CutC/D have been identified in the human gut. These strains include Anaerococcus hydrogenalis, Clostridium asparagiforme, C. hathewayi, C. sporogenes, E. fergusonii, Proteus penneri, and Providencia rettgeri (61).
The rise in circulating TMAO was linked to an increase in pro-inflammatory cytokines TNF-α and IL-1β, alongside a decrease in the anti-inflammatory cytokine IL-10 (43). Trimethylamine N-oxide has the potential to exacerbate atherosclerosis by disrupting cholesterol transport and the formation of foam cells. Additionally, it may increase platelet reactivity, thereby facilitating thrombosis and acute coronary incidents (62). The heightened presence of Escherichia/Shigella was positively associated with the increased plasma levels of TMAO, a metabolite produced by GM that plays a role in the development of CVD (63). Trimethylamine N-oxide may inhibit reverse cholesterol transport (RCT), leading to the accumulation of cholesterol in the arteries and the progression of atherosclerotic lesions (43).
Elevated levels of microbial TMAO induced by diet may enhance platelet activation in response to sub-maximal stimuli such as thrombin, adenosine diphosphate (ADP), and collagen. This condition can also trigger the release of intracellular calcium, leading to increased platelet hyperresponsiveness (64). In clinical outcome studies involving substantial participant cohorts, findings revealed a positive correlation between circulating TMAO levels and heightened risks of major adverse cardiovascular events, mortality rates, and arterial infarction (65). The threshold level of plasma TMAO identified in numerous studies for predicting the risk of all-cause mortality is greater than 6 μM (60). Elevated plasma concentrations of TMAO have been demonstrated to predict the occurrence of MI, stroke, and overall mortality across multiple independent cohorts from both the United States and Europe. Additionally, high levels of TMAO are associated with unfavorable clinical outcomes in patients with HF (66).
Trimethylamine N-oxide concentrations are also increased in various fish and seafood, which are regarded as beneficial for heart health. Notably, the US cohort exhibited somewhat elevated overall TMAO levels in the research conducted by Li et al. (as cited by Papandreou et al.), potentially indicating dietary variations between the two study groups. This suggests that the prognostic significance of TMAO may differ across populations, influenced by their respective dietary practices (59). Trimethylamine N-oxide levels may be influenced by renal and hepatic function, with the kidneys playing a crucial role in the elimination of TMAO, while the liver is involved in the oxidation of TMA (67). A strong adherence to a Mediterranean diet was linked to reduced urinary TMAO levels, regardless of the individual's background diet, whether it be vegan, vegetarian, or omnivorous (68).
14. Trimethylamine Inhibitors
The clinical significance of TMAO may be enhanced by the recent development of TMA inhibitors, which have been shown in animal models to impede the microbial synthesis of TMA. This approach, referred to as "drug the bug", effectively lowers TMAO levels and reverses atherosclerosis in mice, seemingly without eliminating the microbes involved (69).
15. Bile Acids
The metabolism of bile salts and the conversion of cholesterol to coprostanol by the GM can both affect blood cholesterol levels (70). Bile acids are steroids that are both hydroxylated and saturated, playing a crucial role in the emulsification and intestinal absorption of dietary fats and fat-soluble substances (71). Bile acids that are not reabsorbed can undergo deconjugation through the action of bile salt hydrolases (BSHs), which are enzymes produced by various commensal gut bacteria. These bacteria include gram-positive species such as Bifidobacterium, Clostridium, Enterococcus, and Lactobacillus, as well as the gram-negative genus Bacteroides (72). Microbial metabolites, including branched-chain amino acids (BCAAs) and secondary BAs, have the potential to disrupt various metabolic pathways, thereby playing a role in the development of obesity and insulin resistance (62). Primary BAs are synthesized from cholesterol in the liver and subsequently converted into secondary BAs in the gastrointestinal tract. These secondary BAs play a crucial role in regulating various metabolic pathways through receptor signaling and by influencing the composition of the GM (73).
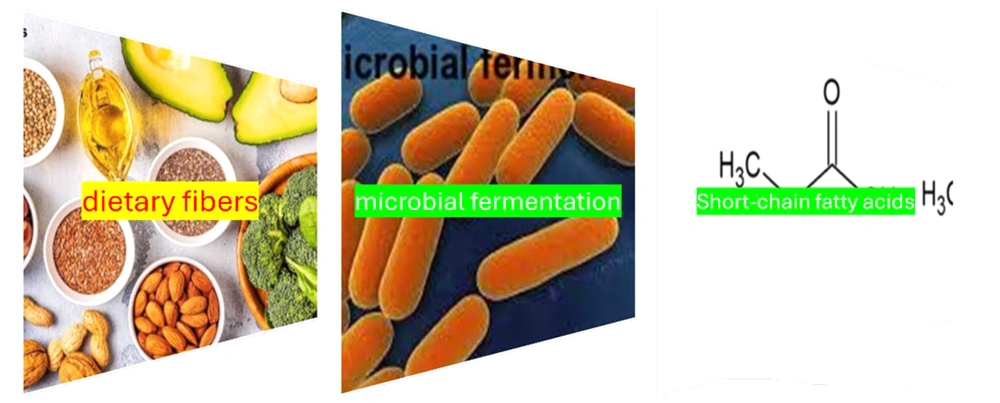
16. Short-Chain Fatty Acids
Short-chain fatty acids are the byproducts of the microbial fermentation of dietary fibers. They play a crucial role in energy extraction from the gastrointestinal tract and are essential for preserving the integrity of the gut mucosa (Figure 2) (74). The bacterial synthesis of SCFAs has the potential to disrupt energy absorption, thereby contributing to obesity, while also affecting gut barrier integrity, which may facilitate the translocation of LPS into visceral adipose tissue (75). The decrease in specific SCFAs, especially butyrate, can lead to an impaired gut mucosal barrier. This impairment may allow for the passive translocation of microbial toxins, including LPS, from the gastrointestinal tract into adjacent adipose tissue and the bloodstream, thereby initiating inflammation and promoting insulin resistance (73). The LPS may also be actively transported across the intestinal membrane in conjunction with triglyceride-rich entities, such as chylomicrons (73). Certain patients with coronary artery disease (CAD) and stroke have been found to exhibit an increased prevalence of potentially beneficial bacteria, including Lactobacillales and Akkermansia, which correlates with a reduction in other SCFA producers (76).
17. Probiotics and Prebiotics
Probiotics offer a potential therapeutic approach for preventing the formation, progression, and eventual degradation of atherosclerotic plaque (77). A meta-analysis indicated a notable reduction in cholesterol, LDL, and triglyceride levels through the use of probiotics (78). The primary mechanism implicated in this process is microbial BA metabolism. Research has shown that the oral administration of probiotics may lead to a reduction in cardiomyocyte fibrosis, cardiac hypertrophy, and the autophagy-signaling pathway in obese rats subjected to a HFD (79).
Probiotics that suppress leptin, specifically L. plantarum 299v, modify the GM, leading to a reduction in circulating leptin levels. This alteration enhances tolerance to cardiac ischemia and mitigates acute injury during ischemia/reperfusion (I/R), as well as reduces hypertrophy and cardiac remodeling following MI (80). A recent meta-analysis examining the impact of probiotics and their derived products on blood pressure revealed an enhancement in blood pressure regulation through multiple mechanisms. A decrease in total cholesterol, LDL, blood glucose levels, and insulin resistance, along with the regulation of the renin–angiotensin system through the synthesis of inhibitory peptides (Val-Pro-Pro and Ile-Pro-Pro), represents several of the underlying mechanisms (81).
The microbiome inhibits or postpones the progression of HF following an infarction. A healthy GM has the ability to regulate immune responses and diminish the size of MI as well as the severity of HF following an MI (82). Insufficient visceral perfusion in individuals with HF may result in ischemia and intestinal edema, which facilitates the translocation of bacteria and their metabolites into the bloodstream via a compromised gut barrier. This process can trigger both local and systemic inflammatory responses. Additionally, metabolites produced by gut microbes have been associated with the pathology of numerous diseases (83).
The symbiotic relationship within the gut serves as a valuable ally, mitigating the risk factors linked to MI and the subsequent occurrences that follow such an event.
18. Medication and Gut Microbiota
Medication represents another crucial element that may lead to changes in GM. Research has indicated that both individual medications and combinations of medications, along with the dosage of these drugs, are significantly associated with alterations in the GM (84). Numerous non-antibiotic medications have the capacity to alter the composition and functionality of microbiota, thereby impacting health outcomes. A notable example is proton pump inhibitors, which rank among the most prescribed medications. These drugs can change the GM, resulting in reduced colonization resistance to enteric infections, such as C. difficile infection, and contributing to the oralization of the colonic microbiota.
Numerous antidiabetic medications, including metformin and liraglutide, achieve their therapeutic effects and provide supplementary metabolic advantages by altering the composition and metabolism of GM. Among the newly developed sodium/glucose cotransporter 2 inhibitors (SGLT2i), empagliflozin has demonstrated a positive impact on enhancing the richness and diversity of the GM, as well as improving inflammatory markers. A recent investigation revealed that empagliflozin facilitated the proliferation of SCFA-producing bacteria, including Roseburia, Faecalibacterium, and Eubacterium, while suppressing the growth of potentially harmful bacteria such as Escherichia-Shigella, Bilophila, and Hungatella. The primary constituents of the intestinal microbial community following dapagliflozin treatment were identified as Muribaculaceae and Lactobacillaceae, whereas Muribaculaceae and Erysipelotrichaceae were linked to MI.
Antihypertensive drugs, including the angiotensin-converting enzyme inhibitor captopril, have demonstrated positive effects on gut pathology related to hypertension, notably by decreasing intestinal permeability, reducing the thickness of the muscularis layer, and enhancing villi length by 55%. Rifaximin demonstrates anti-inflammatory and eubiotic properties, leading to a beneficial modulation of the GM while decreasing the adherence, internalization, and translocation of intestinal bacteria.
Pharmacomicrobiomics is also utilized in the realm of cardiovascular health. Notably, it has been observed that digoxin, a medication prescribed for HF, proves ineffective in approximately 10% of patients. This ineffectiveness is attributed to the conversion of digoxin into its inactive metabolite, dihydrodigoxin, by the bacterium E. lenta (85).
19. Influence of Oral Aspirin on the Gut Microbiome
The use of aspirin increases intestinal permeability and facilitates the entry of LPS into the peripheral bloodstream (86). Aspirin-induced alterations in the microbiome, particularly involving Parabacteroides goldsteinii, may play a role in sustaining intestinal homeostasis, which is disrupted by the administration of aspirin (87). Aspirin contributes to the restoration of GM dysbiosis by enhancing the prevalence of Bacteroidetes, reducing the Firmicutes to Bacteroidetes (F/B) ratio, and increasing the concentrations of SCFAs and beneficial BAs. The atheroprotective properties of aspirin, along with its positive impact on the immuno-inflammatory profile, can be partially ascribed to its influence on GM regulation (88).
20. Influence of Statins on the Gut Microbiome
Statins may provide advantages to the host by influencing the gut microbiome in patients with acute coronary syndrome. Statins have the potential to decrease the presence of potentially harmful bacteria, including P. merdae, while simultaneously promoting the growth of beneficial bacteria such as Bifidobacterium longum subsp. longum, Anaerostipes hadrus, and Ruminococcus obeum (89). Variations in the efficacy of statins may be associated with specific strains of GM that are capable of producing SCFAs and secondary BAs. These compounds can influence the effectiveness of statins by modulating the functions of proteins related to cholesterol metabolism. It is anticipated that metabolites associated with SCFAs and secondary BAs present in the gut will serve as biomarkers for assessing the efficacy of statin treatments (90).
Rosuvastatin seems to enhance the proliferation of anti-inflammatory bacteria while diminishing the presence of pro-inflammatory bacteria. In contrast, atorvastatin may exhibit varied effects on both pro-inflammatory and anti-inflammatory bacteria, in addition to increasing the levels of Bacteroides (91). Alterations in microbiota induced by drugs were linked to the severity of diseases or their outcomes, either directly or indirectly through the metabolism of fatty acids and prenol lipids (92). The utilization of statins may result in changes to the GM and intestinal metabolites, including the BA pool and SCFAs. Conversely, research has shown that the composition of the host's GM can affect the bioavailability of statins in vivo (93).
Statin therapy may provide advantages for patients with acute coronary syndrome by influencing the composition and functionality of the gut microbiome, potentially leading to enhanced levels of circulating metabolites and a decrease in metabolic risk.
21. Phenylacetylglutamine Produced by Gut Microbes
Phenylacetylglutamine (PAGln), produced by gut microbes, serves as an endogenous allosteric modulator of β2-adrenergic receptors. The PAGln, a metabolite produced by GM, has been clinically correlated with and mechanistically associated with CVD and HF (94). Phenylacetylglutamine may represent a promising therapeutic target in HF. The development of effective pharmacological agents to inhibit or diminish the levels of PAGln is essential for examining the impact of such inhibition on cardiac regeneration and, ultimately, its prognosis (95).
22. Bacterial Toxins and Cardiac Function
Bacterial toxins can significantly affect the functioning of the heart. Numerous bacterial toxins can adversely impact cardiac health. Toxins that interact with the membrane, including pore-forming toxins (PFTs) and those that influence membrane receptors, can modify heart function by altering membrane permeability and excitability. This alteration can result in an influx of Ca²⁺ and disrupt Ca²⁺ homeostasis, which is essential for proper heart function. Additionally, the binding of the B-subunits of labile toxic subunit B to gangliosides on cardiac cell surfaces may induce changes in cellular functions, potentially affecting the entire heart and contributing to arrhythmias and sudden cardiac death.
23. Conclusions
The GM can be viewed as a dynamic environmental factor, as its composition is shaped by host genetics, dietary patterns, hygiene practices, and lifestyle choices. This variability positions it as a potential therapeutic target for CVD. The gut microbiome functions similarly to an endocrine organ, producing active biometabolites such as SCFAs, TMA and its oxidized form, TMAO, as well as BAs, thereby influencing the physiological processes of the host. Gut dysbiosis may contribute to the progression of atherosclerosis. The symbiotic relationship within the gut serves as a valuable ally, mitigating the risk factors linked to MI and the subsequent complications that may arise following an MI.