1. Background
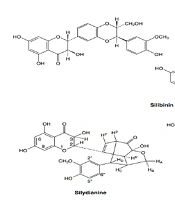
Flavonoids are a family of benzo gamma-pyrones, of which more than 4,000 different flavonoids have been identified to date. These compounds are abundant in both the animal and plant kingdoms, especially in photosynthetic cells. They have been used for centuries for their numerous therapeutic properties. Compounds such as quercetin, taxifolin, and silymarin have been used as active ingredients, both alone and as components of complex chemical preparations (1-3). This compound is extracted from the seeds and fruits of Silybum marianum (Compositae) due to its well-defined therapeutic properties and is actually a mixture of three structural components: Silibinin, silidianin, and silychristin (Figure 1) (3-5). Silymarin is a flavonolignan that has recently been identified as a hepatoprotective agent (3, 6, 7). Silymarin is used to treat many liver disorders (1, 3, 8). Flavonolignans in Silybum marianum (silymarin) protect liver cells by preventing cytotoxins from entering the cells (2, 8, 9). This compound protects liver cells from damage caused by ischemia, radiation, iron overload, and viral hepatitis (3). Silymarin has also shown antihyperglycemic, anti-inflammatory, cardioprotective, and antioxidant effects (3, 6, 9), as well as cytoprotective, hypoglycemic, hypocholesterolemia, anticarcinogenic effects, gastro-protective, and/or anti-ulcer properties (5, 10).
The three structural components of silymarin (3)
The mechanism of action of silymarin involves inhibiting the absorption of toxins such as phalloidin or α-amanitin, preventing them from binding to the cell surface and inhibiting membrane transport systems. In addition, by interacting with the lipid components of the cell membrane, it can affect their chemical and physical properties (3). Carcinogenesis is a process involving steps that are activated by changing the expression of transcription factors and proteins involved in proliferation, cell cycle regulation, differentiation, apoptosis, angiogenesis, invasion, and metastasis (1, 6). Accordingly, factors that can disrupt this cycle are effective anticancer agents (5). Silymarin is able to disrupt this balance by interfering with the expression of cell cycle regulators and proteins involved in apoptosis. Also, the anti-inflammatory and anti-metastatic activity of silymarin is due to the modulation of specific proteins (1, 6, 7).
1.1. The Effect of Silymarin on the Aromatase Enzyme
Aromatase is an enzyme that catalyzes the final step in estrogen biosynthesis. The regulation of aromatase activity and levels of estrogens determines the levels of estrogens, which subsequently have endocrine, paracrine, and autocrine effects on tissues (11). Estrogen is a steroid hormone that maintains female characteristics in the body. In addition to the reproductive system, estrogen plays an important role in the cardiovascular system and musculoskeletal system (12), regulating reproduction, metabolism, behavior, and brain function (11, 12). In the brain, cell survival and neuronal activity are influenced by estrogens and, as a result, aromatase (11). However, estrogen production itself affects female diseases. Therefore, its inhibition is effective in treating diseases (12). The aromatase reaction is the final step in the estrogen biosynthetic pathway, and inhibition of this final step inactivates the biosynthesis of other steroid classes (12). The aromatase enzyme plays a key role in the pathogenesis of polycystic ovary syndrome (PCOS) (13), breast cancer (12), and endometriosis (14). For example, aromatase converts precursor steroids to estradiol and estrogen and promotes the proliferation of endometrial tissue. Therefore, aromatase contributes to disease progression by stimulating an inflammatory response (15). As a result, inhibition of the aromatase enzyme may provide an effective way to treat and control endometrial tissue proliferation by reducing estrogen production (15). Silymarin, which is extracted directly from the dried seeds of S. marianum and has anti-inflammatory (4, 13), antioxidant (6, 16), and anti-fibrotic properties, acts as the main ingredient (13). Silymarin is effective in treating endometrial lesions with antioxidant and anti-inflammatory effects (15) and plays its antioxidant role by eliminating free radicals as well as increasing the levels of glutathione peroxidase and superoxide dismutase (SOD) (6, 10, 16).
1.2. The Effect of Silymarin on HIV
Human immunodeficiency virus-1 (HIV-1) has infected nearly a million people worldwide. Antiretroviral therapy (ART) has now prolonged the lives of people with chronic infection (17). Herbal use is common among HIV patients, often used as self-medication without medical supervision (18). Silymarin extract contains silibinin, which is a mixture of the flavonolignans silybin A (SA) and silybin B (SB). Silibinin has a wide range of antioxidant, immunomodulatory, antiproliferative, antifibrotic, and antiviral activities in tissues and organs. Silymarin and silibinin both inhibit HCV infection in cell culture by variably blocking viral entry, viral fusion, viral RNA and protein synthesis, HCV N5SB RNA-dependent RNA polymerase activity, and virus transmission (17). Although herbal supplements are often harmless, they can actually modulate various cytochrome P450 enzymes (such as CYP3A4) and drug transporters [such as P-glycoprotein (P-gp)], providing evidence for herbal-drug interactions. The most well-known herbal remedy is St. John's wort (Hypericum perforatum), which has drug interactions with and is a potent inducer of CYP3A4 and P-gp activity (18). Laboratory studies have shown that silymarin may interact with many drugs, including HIV protease inhibitors. However, clinical studies with milk thistle have shown conflicting results regarding its ability to modulate these proteins in vivo, and evidence of interactions between milk thistle and antiretroviral drugs from clinical trials has prevented its current use in treatment regimens (18).
2. Objectives
The main objective of this research is to utilize the Molegro Virtual Docker (MVD) software to explore the potential of silibinin, silidianin, and silychristin molecules as ligands within the active site of the Aromatase enzyme and HIV-1 reverse transcriptase and examine the interactions formed by each ligand with other amino acids present in these sites.
3. Methods
Initially, the ligands silibinin (ID = 31553), silidianin (ID = 11982272), and silychristin (ID = 441764) were obtained in the form of structure-data files (SDFs) from the website https://pubchem.ncbi.nlm.nih.gov/. Subsequently, the website http://www.rcsb.org/ was employed to search for the HIV-1 reverse transcriptase and Aromatase enzymes, identified by IDs 1JLQ and 3EQM, respectively. Afterwards, to conduct the molecular docking analysis, version 6 of MVD was installed on a Windows operating system. To perform the docking process, the downloaded proteins and ligands were first entered into the software. Water molecules were eliminated, polar hydrogen atoms were introduced, and energy minimization was conducted. Afterwards, the option for molecule preparation was accessed in the preparation tab. In the Assign all Below section, the setting was modified to "if missing," which allows the software to correct any structural errors in the molecule that may interfere with the docking process. Following that, in the same section, the option for detecting cavities was selected, leading to the choice of the molecular surface in the expanded Van Der Waals mode. The maximum number of cavities was established at 100 to pinpoint the most extensive areas available for the molecular-level docking process, and the cavities associated with the main ligand of each molecule were selected. For the docking procedure, the binding sites of the principal ligands were chosen: The 76.288 cavity for the HIV enzyme and the 304.64 cavity for the aromatase enzyme. Subsequently, the Docking wizard part was chosen from the Docking section of the software, and the imported ligands were selected to configure the parameters associated with the docking procedure. The score was calculated using the MolDock Score (GRID) mode, with the origin in the binding site section configured to the designated cavities. The algorithm employed for this procedure was MolDock simplex evolution (SE), and ten runs were conducted for each ligand. Ultimately, the software was instructed to save the poses file in Mol2 format. After the docking procedure was completed, the outcomes were reviewed (19, 20).
4. Results
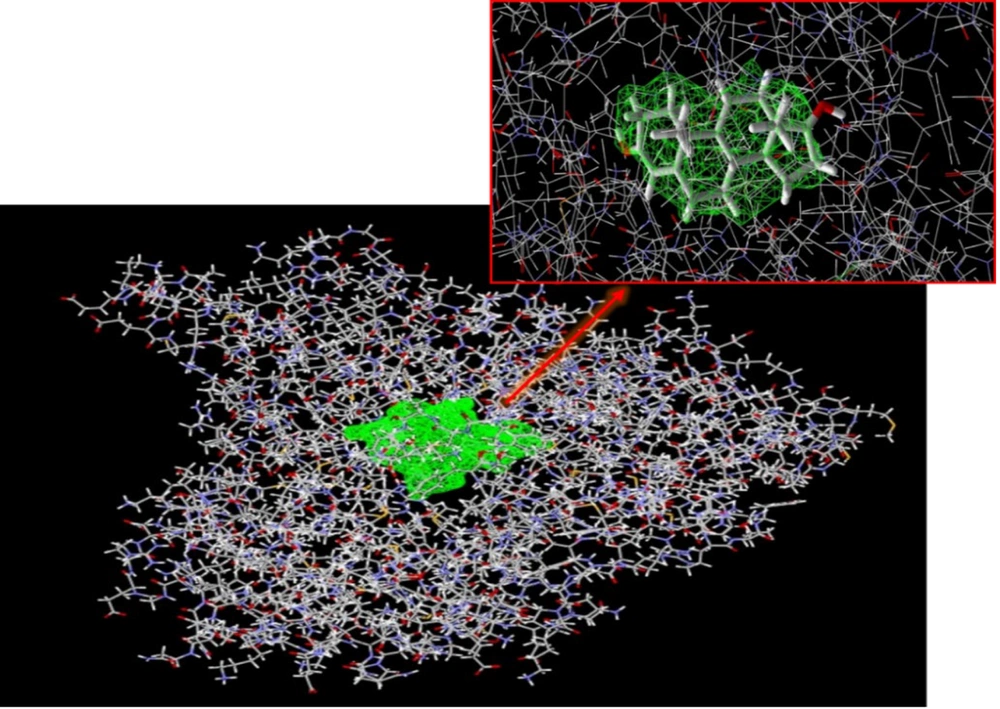
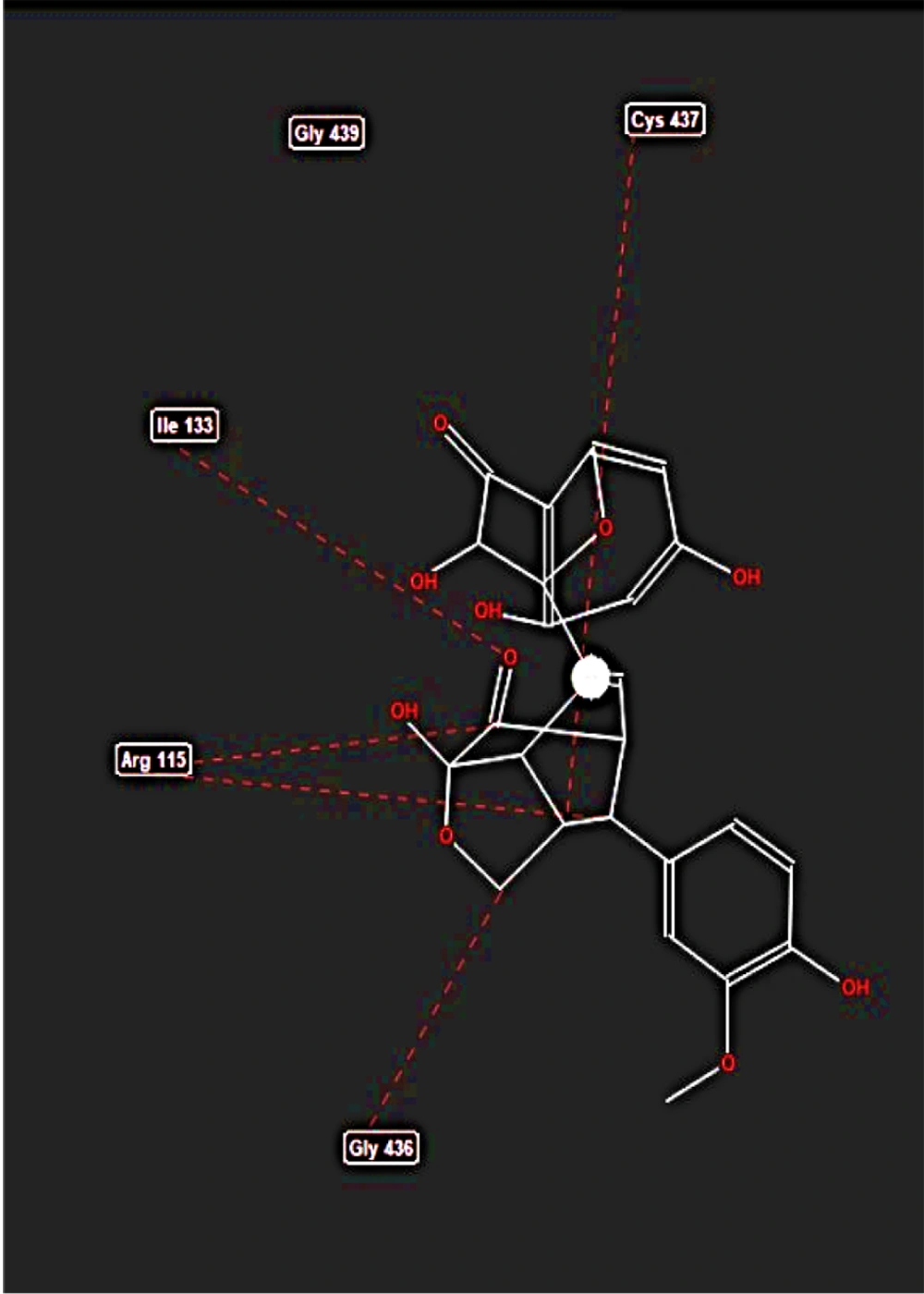
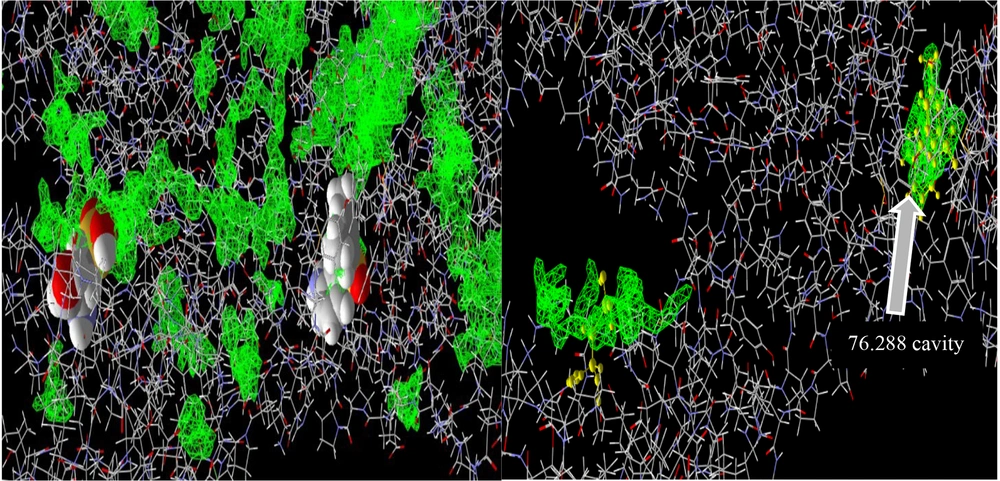
The active site of the aromatase enzyme was identified through its native ligand. This cavity was measured by MVD, which perfectly accommodates the active site (Figure 2). After the docking process, the results show that silibinin possesses considerable potential to bind effectively within the aromatase active site by hydrogen and steric interactions (Figure 3), with a total energy measurement of -141.972 kcal/mol (Table 1).
| Descriptors | MolDock Score | Rerank Score |
|---|---|---|
| Silibinin | -141.972 | -79.431 |
| Siladianin | -127.805 | -105.274 |
| Silychristin | -148.425 | -113.008 |
MolDock Score and Rerank Score of Each Ligand with Aromatase Enzyme
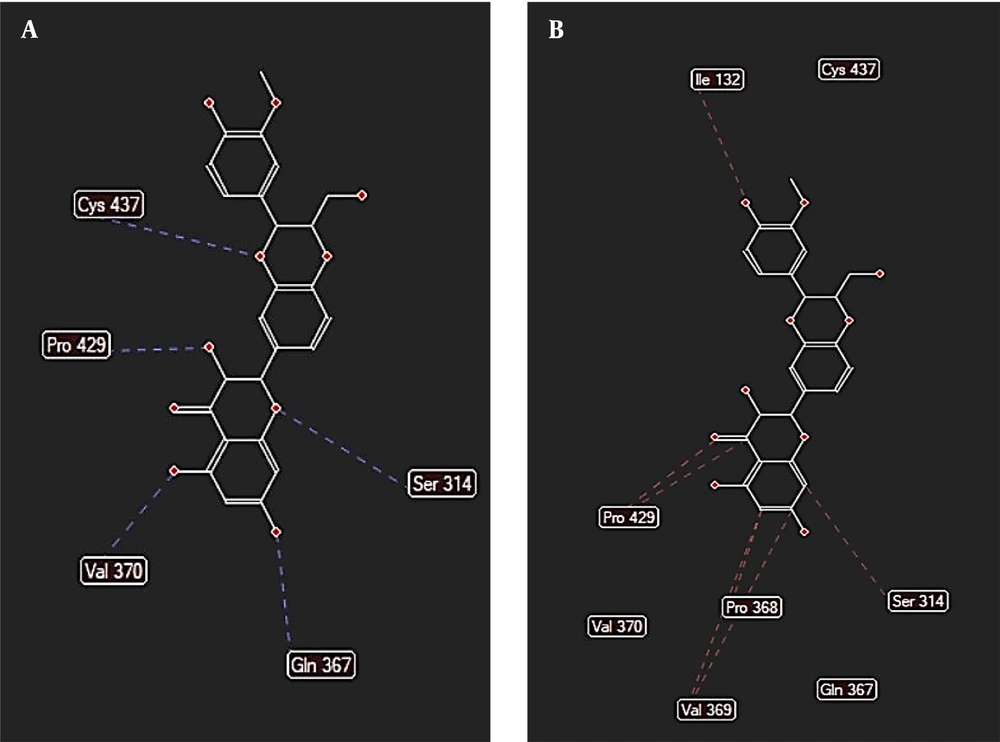
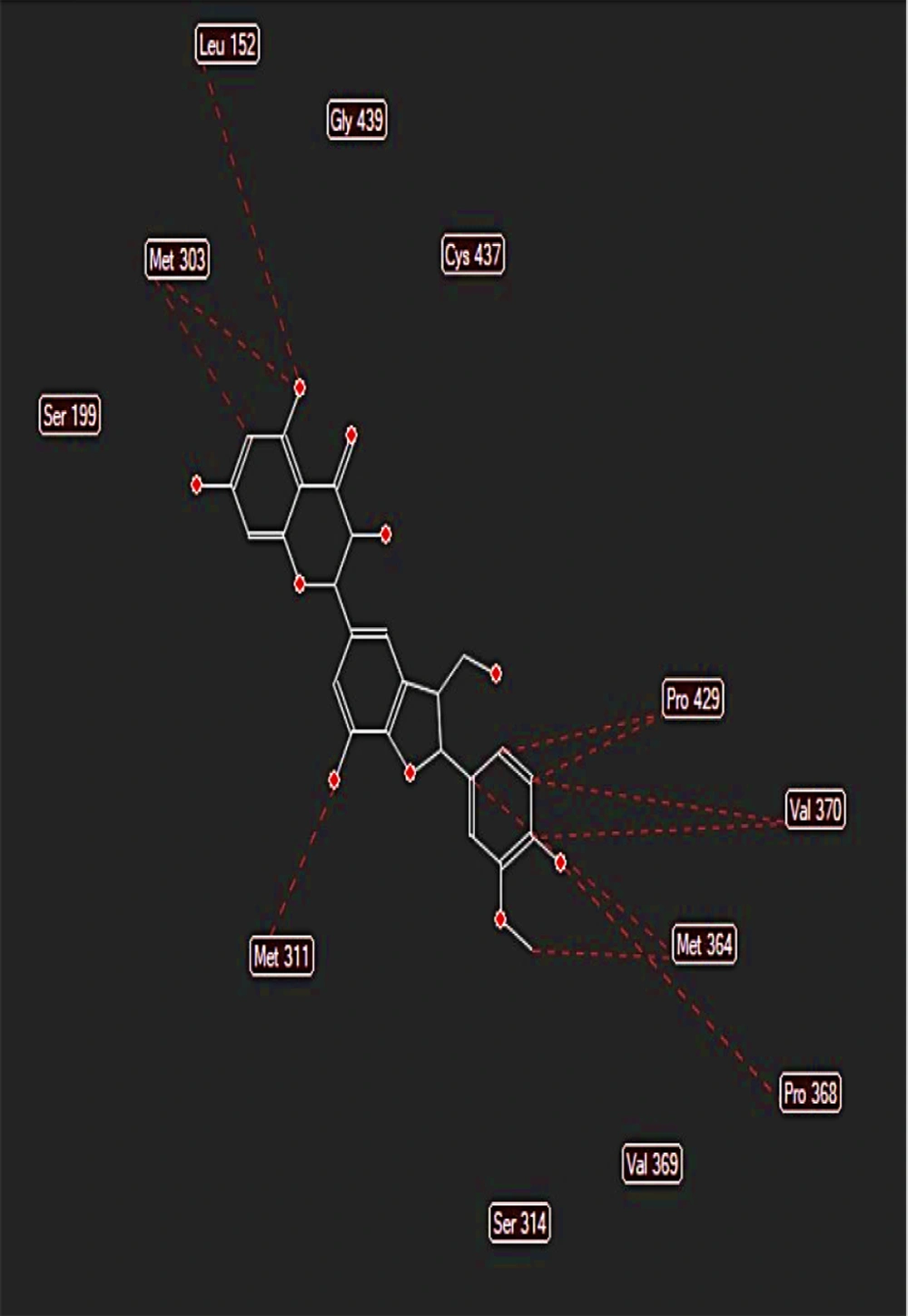
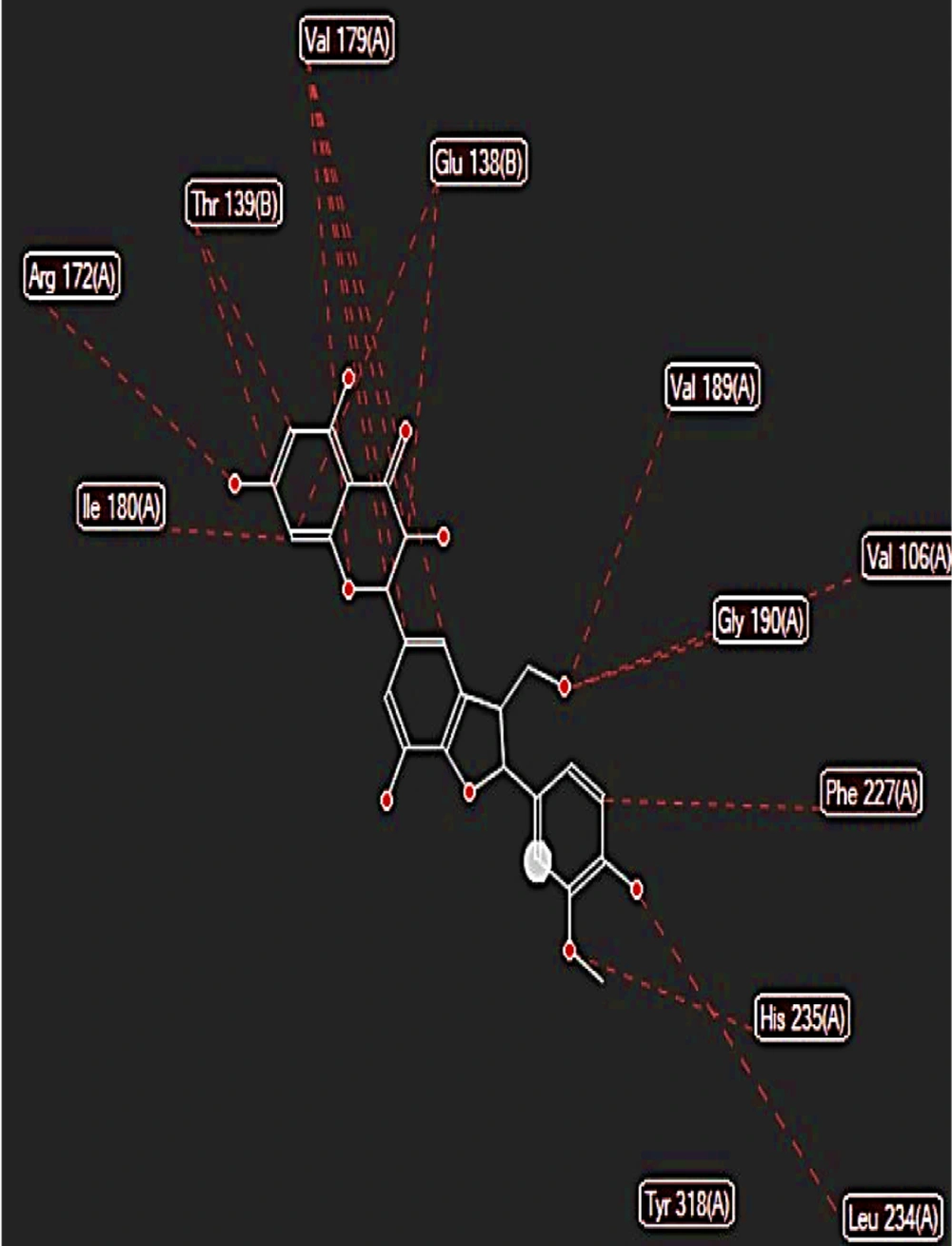
The binding free energy of silychristin at the active site of the aromatase enzyme was computed using software, yielding a value of -148.425 kcal/mol (Table 1). This result suggests that silychristin exhibits a strong affinity for the active site, allowing it to compete effectively with the primary ligand for binding. The high binding affinity is attributed to the establishment of hydrogen bonds with the amino acids cysteine 437, valine 369, serine 314, and methionine 303, along with the potential formation of steric interaction bonds with other amino acids (Figure 4), which may further enhance the binding strength.
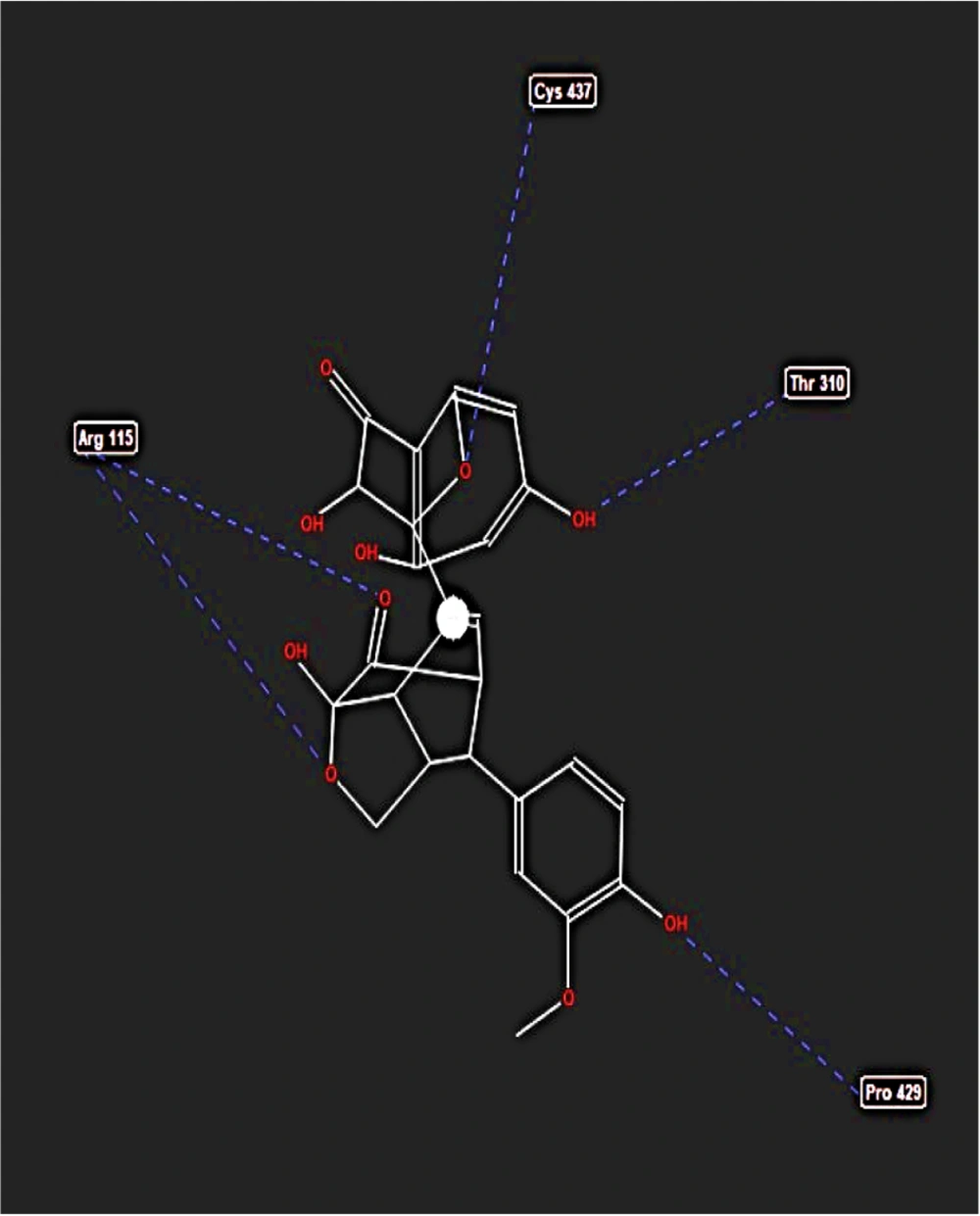
During the molecular docking analysis of the silidianin ligand within the active site of the aromatase enzyme, it was found that this compound exhibits a notable capacity to bind to the active site as a ligand, achieving a binding energy of -127.805 kcal/mol in this site (Table 1). Figures 5 and 6 reveal the amino acids that play a crucial role in forming hydrogen and ester bonds.
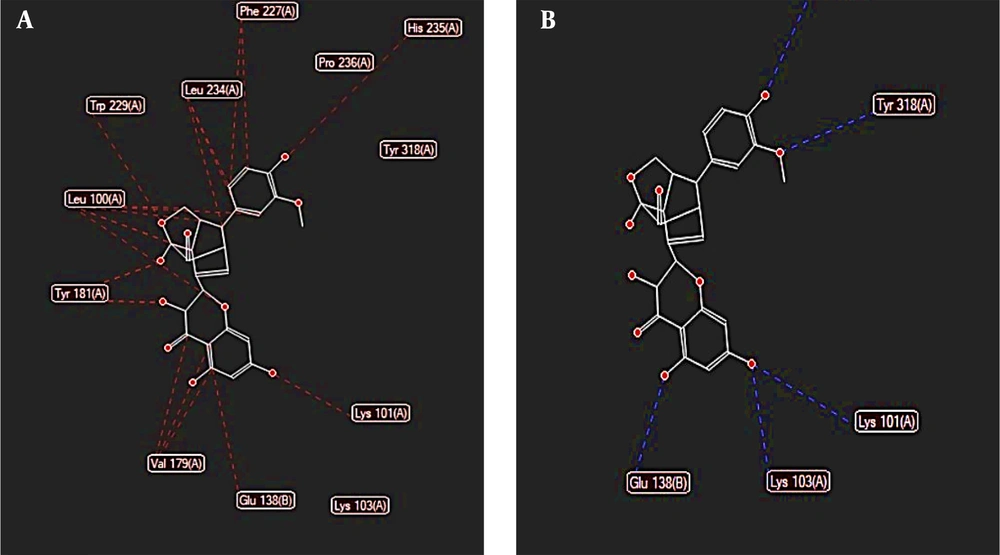
During the docking procedure with the HIV-1 reverse transcriptase molecule, the software identified 41 potential cavities, among which the 76.288 cavity, recognized as the active site for the primary ligand of this enzyme, was chosen (Figure 7). All three molecules demonstrated considerable binding potential at this site. The findings indicated that the silibinin molecule could occupy this site with a free energy of -140.701 kcal/mol, establishing hydrogen bonds with the amino acids Ile 180, Tyr 318, and Arg 172 in the enzyme's A chain. Additionally, the silidianin molecule exhibited promising results, achieving a binding energy of -138.956 kcal/mol (Figure 8). Lastly, silychristin also showed effective binding by forming hydrogen bonds with Tyr 318 in the A chain and Glu 138 in the B chain, along with other ester bonds in this site (Figure 9), resulting in a free energy of -135.365 kcal/mol (Table 2).
| Descriptors | MolDock Score | Rerank Score |
|---|---|---|
| Silibinin | -140.701 | -92.856 |
| Siladianin | -138.956 | -16.734 |
| Silychristin | -135.365 | -67.577 |
MolDock Score and Rerank Score of Each Ligand Human Immunodeficiency Virus-1 Reverse Transcriptase Molecule
5. Discussion
The MVD is a molecular docking software utilized in numerous studies owing to its exceptional accuracy, affordability, and rapid processing capabilities (21). This software can automatically detect potential ligand binding sites, which can be modified within the software settings (20). Furthermore, users can select between two algorithms, the MolDock optimizer and the MolDock SE, based on their specific requirements. The primary distinction between these algorithms lies in the capacity to recombine poses, a feature that is exclusive to the SE mode. Users have the option to utilize the if-missing statement to rectify potential inaccuracies in their ligand or receptor structures. This approach was employed in the current study, despite the absence of any errors in the ligand and enzyme structures examined.
Recognizing the significance of this software, Kusumaningrum et al. utilized it to conduct molecular docking of NO11 (a modified naphthoquinone) as a potential inhibitor of Polo-like kinase 1 (Plk1), which is associated with various cancers. Their findings indicated that NO11 did not establish any hydrogen bonds with Plk1. Nevertheless, the software calculated that the MolDock score for compound NO11 is -134.73 kcal/mol, attributed to the steric interaction bonds formed with Phe 133, Asp 194, Glu 101, Lys 82, Cys 133, and Glu 140 of Plk1 (20).
In a separate research study, Naeem and associates examined chlorogenic acid as a ligand targeting aldose reductase, a crucial enzyme in the polyol pathway that aids in the transformation of glucose into sorbitol with the involvement of NADPH, which is linked to complications arising from diabetes (19, 22). The findings indicated that chlorogenic acid is capable of establishing hydrogen bonds with the amino acids Tyr 48, His 110, and Trp 111 located in the enzyme's active site. The average MolDock score for chlorogenic acid was -119.34 kcal/mol, while the MolDock Re-rank score was estimated at -114.92 kcal/mol (19).
5.1. Conclusions
This research utilized three molecules — silibinin, silidianin, and silychristin — derived from the seeds and fruits of silymarin (3-5), as effective ligands to explore their potential binding effects on HIV-1 reverse transcriptase and the Aromatase enzyme. Numerous studies have demonstrated that silymarin offers protection to liver cells from ischemic damage and radiation exposure (3). Furthermore, it has exhibited antioxidant properties in various investigations (3, 6). Consequently, this study employed these compounds in a docking process, revealing their potential as suitable ligands for binding to the active sites of both HIV-1 reverse transcriptase and the Aromatase enzyme, thereby destroying their enzymatic activities. The inhibition of the Aromatase enzyme, which is crucial in the context of cancer cell proliferation, could serve as a foundation for developing drugs based on silymarin compounds. Additionally, these molecules may facilitate the creation of potent medications aimed at inactivating the HIV-1 reverse transcriptase in infected cells, thereby preventing viral replication and addressing the global HIV epidemic, which affects approximately one million individuals. While the findings of this study suggest that these molecules can bind to the active site of the enzymes, additional in vivo research is necessary to verify the accurate binding of these ligands.