1. Background
Motor learning is a set of processes associated with practice or experience leading to relatively permanent gains in skilled performance (1). Fitts and Posner (2) proposed three phases of motor learning and described the earliest, the cognitive stage, as being characterized by a high degree of conscious regulation of movement. Gradually, verbal-cognitive involvement decreases, and the performer reaches the final stage of skill development, the autonomous stage. During this stage, movements become more stable and efficient with improved sensorimotor processing and execution.
Advancing from Fitts and Posner’s stages of motor skill acquisition (2), Schmidt (1) proposed the concept of “general motor programs” (GMPs), whereby the learner develops an abstract representation for classes of movements (walking, running, throwing). Schmidt’s (1) theory allowed for many different variants of a general type of movement to be produced by the same program by specifying new parameters (velocity, force, direction) based on the context in which the skill is performed. Based on GMP theory, practice schedules that randomize tasks (contextual interference or CI) and/or those that employ variable practice, in which consecutive repetitions of a task differ through variations or unpredictability, are more cognitively engaging leading to better skill retention and transfer (3).
Electroencephalography (EEG) is a proven method for examining psychomotor states during motor learning and performance (4-6). There are distinct functional roles for the various EEG frequencies found in the brain, with particular importance of alpha (α; 7 - 13 Hz) and beta (β); 14 - 30 Hz) oscillations for motor planning and execution (7, 8). Because different cortical regions relate to specific cognitive and motor functions, topographical assessment of EEG can be employed to infer various psychomotor processes occurring during motor skill acquisition (9).
One technique used in analyzing EEG frequencies is event-related desynchronizations (ERDs) and synchronizations (ERSs), which in the α frequency may be interpreted as a correlate of cortical activation (ERD) and cortical deactivation (ERS) (10). When an individual is engaged in a task requiring focused attention, a decrease in α power is observed in brain regions that are task relevant and an increase in α power is observed in brain regions that are task irrelevant (11). One may think of α power as modulating cortical activity by either inhibiting enhancing cognitive processing. Considering the well-established inverse relation between α power and cortical activation, as skill level increases, one observes greater efficiency in execution of motor skills, which is manifested in an attenuation in α ERDs (12).
Although researchers have used EEG to determine cortical adaptations associated with visuomotor performance (9, 13), the preponderance of research has focused on examining differences between novice and expert performers (4, 14, 15) without considering the transition through the stages of motor learning espoused by Fitts and Posner (2). Moreover, it is still unclear as to how cortical oscillations linked with the motor skill change under differing practice schedules. Based on the literature reviewed and the paucity of research investigating neural oscillations occurring during motor skill practice we investigated (1) the behavioral effects of practice on learning, retention, and transfer during repetitive (RP) and variable (VP) practice schedules on an anticipation timing task (ATT); and (2) changes in cortical α (8 - 13 Hz) and β (14 - 30 Hz) oscillations at early (EP) and late (LP) stages of practice.
2. Methods
2.1. Subjects
Fourteen male (n = 6) and female (n = 8) college-age participants, ages 19 - 25 (mean age = 22, SD = +1.48, range = 19 - 25), without visual, central nervous system, and/or musculoskeletal problems involving the dominant (in this investigation right) upper extremity were recruited and equally assigned to RP and VP conditions. Participants were instructed to refrain from drinking alcohol the night before and the day of participation and from drinking caffeine and participating in exercise the day of participation. The study was conducted in compliance with U.S Government and University of New Hampshire standards for research with human subjects.
2.2. Procedures
In the first visit participants (1) completed a ‘Participant Demographic and Screening Questionnaire’ to determine potential visual, central nervous system, and/or orthopedic problems that might compromise their performances on the ATT; (2) gave their informed consent; (3) were fitted for the appropriately sized EEG elastic electrode cap; (4) observed the ATT to be performed; and (5) were assigned to one of the practice conditions.
In the second visit, participants actively engaged in the experimental process. After participants were prepped, they were seated comfortably in an armchair with their moving arm’s thumb positioned over the button of the anticipation-timing apparatus (Bassin Anticipation Timer, Model #35575, Lafayette Instruments, runway length = 539 cm). To measure learning, participants in both groups performed a five-trial pre-practice test at 5.36 m/s., after which they performed 300 practice trials. During the 300 practice trials, participants performed either RP (practicing at a constant velocity of 5.36-m/s) or VP (practicing at random velocities from 4.47 m/s to 35.76 m/s) (Figure 1). As feedback of performance is consequential to learning, knowledge of results was given to participants after each trial during practice according to procedures outlined by Croce et al. (16). Five minutes after performing practice trials, participants performed a five-trial post-practice test at 5.36 m/s (retention) and 30 min later performed two five-trial post-practice tests, one to measure longer retention (5.36 m/s) and one to measure transfer to a novel velocity (22.35 m/s).
A BrainVision 64-channel EEG system (version 2.2) were used to collect and analyze data in accordance with standard international 10-10 system and traditional recording methodology (17). Before each practice trial, a 2 s baseline measurement was taken, which was used as a reference for determining percent changes in power resulting from practice. Baseline was followed by a 2 s cue light and then a stimulus light. The stimulus light denoted the beginning of a series of runway lights that participants followed down the runway. When the light reached the end of the runway, participants anticipated the final stimulus light by pressing a response button, after which they were instructed to relax for 2 s.
2.3. Data Analysis
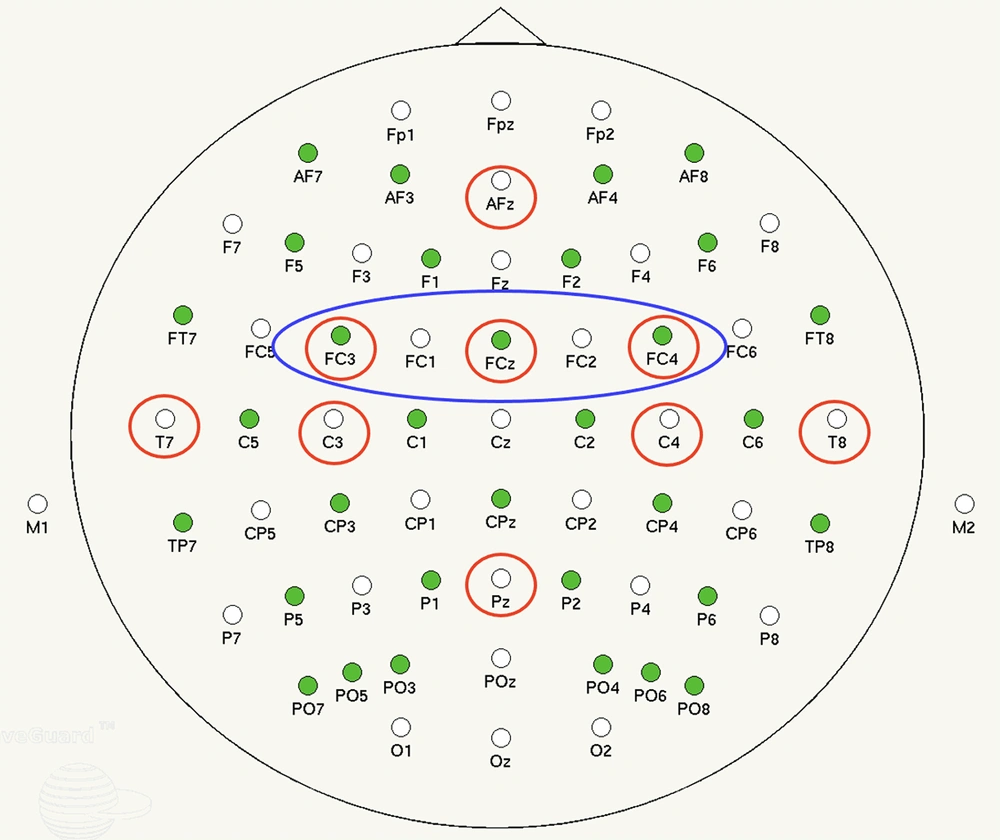
Power analyses focused on cortical areas associated with the frontoparietal network (FPN) and motor learning (18). Based on electrode montages used in previous research (4, 19) electrodes of interest (EOI) were as follows (Figure 2): (1) AFz and Pz (general and task-relevant attentional resources, respectively); (2) T7 (verbal-cognitive processing and memory encoding), T8 (visuospatial processing); (3) left (C3) and right (C4) sensorimotor cortices (motor execution); and (4) left (CP3) and right (CP4) premotor and supplementary (FCz) motor cortices (motor planning).
Raw EEG data were re-reference to a common average. A Butterworth filter (low cutoff, 0.01 Hz; high cutoff, 40 Hz; 12 decimal/octave) was used to filter noise and an independent component analysis (ICA)-based ocular correction was used to remove noise resulting from ocular movements. Data were segmented into trials beginning with a 2 s period preceding the cue light (baseline) and ended 2 s post-response-button press. Baseline was used as the reference interval for determining percent power changes occurring from the cue light through the 2 s post-response-button press.
Practice trials were divided into EP (first 60-trials) and LP (last 60-trails) for data analyses (16). Power was examined in α (10 - 13 Hz) and β (14 - 30 Hz) frequencies. Whilst motor learning is known to exact widespread α frequency changes throughout the cortex (20), β frequency changes take on special significance in prefrontal and sensorimotor cortices (8, 21). Therefore, α frequencies were analyzed over all electrodes and β frequencies were analyzed exclusively over C3, C4, CP3, CP4, FCz, and AFz electrodes. β frequencies were analyzed using beta modulation depth (BMD), which uses ERS-to-ERD peak-to-peak differences (21). As both left and right premotor areas are involved in organizing and planning both left- and right-sided limb movements, CP3 and CP4 (premotor) and FCz (supplementary motor) data were pooled and averaged to represent inclusive premotor activity (PMA).
Alpha and β ERSs/ERDs were analyzed using separate, 2 × 2 repeated measures ANOVA (group [RP and VP] × practice trial blocks [EP and LP]) on EOI. To investigate the effects of practice on motor performance, error data on the ATT was parameterized using root mean square error (RMSE) (8) and analyzed using a 2 × 4 repeated measures ANOVA (group [RP, VP] × test block [pretest, 5 min post retention, 30 min post retention, 30 min transfer]). Greenhouse-Geisser adjustment factor was applied to analyses to correct for inherent correlations of repeated measurements and the Sheffe’s test was used in post hoc analyses. Cohen’s r was applied to all significance statistics to measure size difference between groups.
3. Results
3.1. Behavioral Measures of Motor Skill Acquisition
Behavioral results indicated a significant learning effect for both groups (F (3,27) = 12.68, P < 0.001, r = 0.79), and a significant group × test interaction effect (F (3,21) = 12.68, P ≤ 0.05, r = 0.81), with VP participants performing better on the 30 min transfer test (Table 1 and Figure 2). Results are consistent with the literature on variable practice being more cognitively engaging, leading to better skill transfer to novel conditions and contexts (3).
| Test and Block | Practice Conditions | |
|---|---|---|
| Repetitive Practice | Variable Practice | |
| Pretest | 82.68 ± 38.4 | 124.80 ± 44.8 |
| 5 min post-practice retention | 31.00 ± 13.6 | 31.60 ± 8.7 |
| 30 min post-practice retention | 30.90 ± 5.7 | 38.40 ± 14.0 |
| 30 min post-practice transfer | 80.10 ± 54.8 | 24.60 ± 10.8 |
a Note: There were significant learning (P ≤ 0.0001) and significant group × test interaction (P < 0.05) effects.
b Values are expressed as mean ± SD.
3.2. Alpha Frequencies
For electrode C3 (contralateral motor cortex), there was a significant practice effect (F (1,12) = 185.91, P ≤ 0.001, r = 0.57). For electrode C4 (ipsilateral motor cortex), there were nonsignificant group (P = 0.12) and practice (P = 0.06) main effects. For pooled PMA electrodes, there was a significant practice main effect (F (1,12) = 119.29, P ≤ 0.001, r = 0.61), and a significant group × practice interaction effect (F (1,12) = 4.77, P ≤ 0.05, r = 0.53) (Table 2). For electrode T7 (left-temporal cortex) there were significant practice (F (1,12) = 118.93, P ≤ 0.001, r = 0.71), and group × practice interaction, F (1,12) = 5.12, P ≤ 0.05, r = 0.46, effects. For electrode T8 (right-temporal cortex) there were no significant differences by group (P = 0.61) or practice (P = 0.40) (Table 2). For electrode AFz (central frontal cortex) there was a significant practice effect (F (1,12) = 18.98, P ≤ 0.001, r = 0.62) (Table 2). For electrode Pz (central parietal cortex) there were no significant group (P = 0.50) or practice (P = 0.052) (Table 2).
| Electrode Site; EEG | Practice Conditions | |||
|---|---|---|---|---|
| Repetitive Practice | Variable Practice | |||
| Early Practice | Late Practice | Early Practice | Late Practice | |
| C3 | 80.41 ± 12.58 | 65.57 ± 10.23 | 71.41 ± 10.63 | 58.86 ± 10.86 |
| C4 | 10.48 ± 01.50 | 09.88 ± 01.43 | 09.04 ± 01.62 | 08.76 ± 01.42 |
| PMA (PC3, FCz, PC4) | 67.84 ± 11.51 | 45.48 ± 11.04 | 73.15 ± 13.78 | 58.25 ± 09.60 |
| T7 | 47.30 ± 09.58 | 25.83 ± 06.88 | 48.14 ± 09.63 | 34.05 ± 08.52 |
| T8 | 47.98 ± 11.21 | 46.09 ± 10.07 | 44.21 ± 18.04 | 43.07 ± 17.40 |
| AFz | 44.82 ± 11.01 | 34.03 ± 04.10 | 43.91 ± 08.40 | 30.73 ± 03.80 |
| Pz | 35.95 ± 08.13 | 33.52 ± 11.64 | 39.75 ± 09.72 | 37.15 ± 11.65 |
a Note: For C3 there was a significant practice effect (P > 0.001); for PMA there was a significant practice effect (P > 0.001) and a significant group × practice interaction (P > 0.05); for T7 there was a significant practice effect (P > 0.01) and a significant group × practice interaction (P > 0.05); for AFz there was a significant practice effect (P > 0.01).
b Values are expressed as mean ± SD.
3.3. Beta Frequencies-Modulation Depth
For electrode C3 there was a significant practice effect (F (1,12) = 161.61, P ≤ 0.001, r = 0.63), and a significant group × practice interaction effect (F (1,12) = 8.94, P ≤ 0.05, r = 0.11), with VP participants displaying less of a change than RP participants in LP. For electrode C4, there were no significant group (P = 0.64) and practice (P = 0.15) effects. For the PMA pooled data, there was a significant practice effect (F (1,12) = 73.81, P ≤ 0.001, r = 0.53). For electrode AFz, there was a significant practice effect (F (1,12) = 14.26, P ≤ 0.01, r = 0.54) (Table 3).
| Electrode Site; EEG | Practice Conditions | |||
|---|---|---|---|---|
| Repetitive Practice | Variable Practice | |||
| Early Practice | Late Practice | Early Practice | Late Practice | |
| C3 | 92.68 ± 09.94 | 116.00 ± 16.63 | 98.43 ± 11.41 | 112.26 ± 09.98 |
| C4 | 69.72 ± 11.67 | 65.37 ± 11.91 | 70.23 ± 12.44 | 70.43 ± 08.55 |
| PMA (PC3, FCz, PC4) | 82.84 ± 11.39 | 102.58 ± 19.02 | 92.01 ± 10.44 | 104.97 ± 12.38 |
| AFz | 111.98 ± 17.65 | 146.40 ± 21.97 | 124.51 ± 21.30 | 143.051 ± 22.75 |
a Note: For C3 there was a significant practice effect (P > 0.001) and a significant group × practice interaction (P > 0.05); for PMA there was a significant practice effect (P > 0.001); for AFz there was a significant practice effect (P > 0.01).
b Values are expressed as mean ± SD.
4. Discussion
4.1. Behavioral Measures of Motor Skill Acquisition
There was a significant learning effect with participants in both groups displaying lower error scores on retention and longer retention tests over pretest scores, with VP participants performing significantly better on the novel velocity transfer test. This is consistent with prior research showing that variable practice leads to improved transfer of skills learnt to a novel condition (3, 22) and can be explained by the ‘generalized motor program theory’ as posited originally by Schmidt (1). Unlike practice in constant conditions, practice in variable conditions, involves individuals reacting to variations of a stimulus and must produce one of many variations of the movement learnt, which leads to improved transfer of skills learnt to novel conditions (1, 23).
4.2. Alpha Frequencies and Motor Skill Acquisition
At C3 (contralateral sensorimotor cortex) there was attenuated α ERD during late practice. Attenuated α ERD suggests that the skill was becoming more automated and more efficient, requiring fewer cortical resources. A decrease in α ERD in motor skill practice has been reported previously (24, 25) and has been observed in experts compared to novice performers (26). The PMA, represented by an aggregate of FC3, FC4, and FCz electrodes, is involved in motor planning and preparation. Significant practice and group × practice interaction effects were observed in both group participants, demonstrating less cortical activation over the course of practice; however, there was a greater α attenuation found in RP participants, indicating they displayed greater cortical efficiency in planning and performing the ATT compared to VP participants.
These results are consistent with previous research (24). Research likewise indicates that α ERD is reduced in expert relative to novice performers indicating increased cortical efficiency in motor planning and execution (5, 15). Attenuated α ERD at T7 in the left temporal lobe was found in in both group participants during LP. This decrease in α ERD suggests that less cognitive-verbal rehearsal was needed as the skill became more automated from EP to LP; however, this α attenuation was less pronounced in VP participants.
Less α attenuation at C3, PMA, and T7 in VP participants could be that there was less automaticity performing the task due to the changing velocities encountered during practice trials and thusly required more verbal-cognitive processing and greater cognitive engagement when practicing the ATT. A decrease in cognitive-verbal rehearsal resulting from practice has been reported previously (12, 27), and has been found to a greater extent in expert compared to novice performers (15, 28). According to Dyke et al. (28), verbal-cognitive processing is superfluous for expert performers and may be counter-productive and detrimental to their performances.
Frontal and parietal midline electrodes, representing general and task-specific attentional resources, respectively, provided information on allocation of attentional resources during EP and LP stages. Data from AFz indicated a winnowing of general attentional resources for participants in both groups. It appears that because of practice, there was less reliance on a general allocation of attentional resources as participants were more specifically engaged in viewing the stimuli and coordinating their responses. Conversely, there were no significant differences in α activity over the course of practice at Pz, indicating the necessity for task-specific attention. Hatfield et al. (5) also found no change at the Pz electrode site when investigating novice versus expert pistol shooters.
In total, α ERD results are in accordance with previous research comparing α EEG activation in experts compared to novice performers. These studies indicate that the most accurate performances in experts are associated with attenuated α in premotor, sensorimotor, visuospatial, and attention-related cortical areas during motor preparation and execution (28). Attenuated α activity in skilled performers can be because skilled performers have fostered highly developed motor programs housed in non-cortical, motor brain regions such as the basal ganglia and cerebellum (13). Activity in verbal-analytic brain regions, which are interconnected with motor regions, may introduce unnecessary complexity into an otherwise automatic process occurring in motor and premotor areas. Conversely, motor performance of non-experts and early practice learners is exhibited by more verbal-analytic-related activity (29). Lacking highly developed motor programs, these individuals benefit from verbal analysis from teachers/coaches or without available instructions engaging in ‘self-talk’ regarding performance specifics (30).
Prior research validates that practicing a novel visuomotor task is associated with changes in neural networks involved in attention, motor preparation, and sensory integration in a similar way to that found in expert performers. Decreased activity in these networks during later motor learning stages can be attributed to more efficient cortical processing as participants develop a stronger motor schema (4). This investigation, along with results from previous investigations, presents compelling evidence that cortical activity of early-stage learners is associated with greater levels of verbal analytic and working memory during stimulus processing, motor preparation, and motor execution than late-stage learners, and that less attenuation of α activity in VP participants can be attributed for their need to be more cognitively engaged in the activity.
4.3. Beta Frequencies and Motor Skill Acquisition
The role of movement related beta desynchronization (MRBD) is to filter incoming sensory information during movement and reflects a simultaneous activation of motor areas and an attenuation of sensory areas during movement, whilst subsequent β rebound (PMBS) reflects adaptive changes in the motor program driven by sensory feedback (31), and/or feedforward motor program-model updating (32); therefore, higher amplitude PMBS might be indicative of more confidence in feedforward estimations and the maintenance of a more stable motor output (33) and lower amplitude PMBS might be indicative of low confidence in feedforward estimations and the need for adaptive changes driven by sensory feedback (34).
In the present investigation participants in both groups displayed an overall increase in BMD from EP to LP in C3, PMA, and AFz areas, with VP participants exhibiting less of a change than RP participants (Table 3). Consequently, the lower amplitude BMD found in VP participants in practice might be indicative of low confidence in feedforward estimations and the need for adaptive changes driven by sensory feedback, making it more likely that VP participants were better able to respond to ‘new’ velocity conditions not observed during practice. One might think of the varying velocities under which VP group participants practiced as improving the ‘motor algorithm’ used by the sensorimotor system to adjust the ‘motor action plan’ to suit the ATT under new velocity conditions. Previous research has been mixed as some researchers have observed increased PMBS (34) whilst others have observed attenuated PMBS (35). Likewise, previous research has been mixed regarding MRBD, with some observing increased levels (36) while others observing attenuated levels (8).
It has been posited that β changes recorded over frontal regions subserve several functions supporting motor learning, such as the maintenance of sensorimotor representations, processing of sensory reafference, and visuomotor attention (34). In the present investigation β changes like that found by Tatti et al. (21) were observed in participants in both groups, with no significant difference between groups, albeit increases were greater in RP participants.
Results from our investigation suggest that greater BMD during LP was reflective of neural processes that facilitated practice-dependent changes in frontal and sensorimotor cortices related to motor skill acquisition and are strongly aligned to the hypothesis forwarded by Tan et al. (34), who suggested increased cortical PMBS may be used by the sensorimotor cortex in (1) recalibrating the motor network to new conditions and prepare for subsequent movements; and/or (2) processing new information leading to updating of the ‘estimation uncertainty’ associated with the current feedforward model and revising or maintaining the existing motor program. A decrease in the PMBS following a movement indexes high uncertainty and low confidence in current feedforward estimations, which then allows for more flexibility in the action plan (which was found in VP participants). It is possible that this uncertainty allows for greater flexibility in motor programming when an individual encounters performance conditions.
4.4. Synthesis of Alpha and Beta Frequencies and Motor Skill Acquisition
The combination of reduced sensorimotor, frontal, and left temporal α frequencies observed in LP might be reflective of improved cortical efficiency needed to compute the transformations necessary between the visual representation of the target and performance on the ATT (6). In concert with the attenuated α observed during LP, the greater BMD observed might be reflective of neural processes facilitating practice-dependent changes in the sensorimotor cortex related to skill acquisition and used to recalibrate the motor system to prepare for subsequent movements. The fact that there were greater BMD in RP participants during LP might be indicative of greater confidence in feedforward estimations and the maintenance of a more stable motor output, whilst lower amplitude BMD in VP participants might be indicative of low confidence in feedforward estimations and the need for adaptive changes driven by sensory feedback. Taken together, less attenuation of α ERD along with lower BMD observed in VP participants would permit a more adaptable and flexible motor program.
When individuals engage in variable practice, they experience a range of conditions, such as changes in environment, task complexity, or movement variations. Incorporating variability into motor skill practice enhances motor learning and retention by exposing individuals to diverse conditions during practice, which in turn assists them in developing a more robust and adaptable motor skill repertoire.
The present findings are particularly important for several reasons. Firstly, for supporting the foundational precepts of Fitts and Posner’s (2) and Schmidt’s (1) theories of motor skill acquisition that practice allows for more automated neural processing in cortical areas essential for motor learning and performance. Secondly, for supporting that variable practice is essential for learners to develop a more elaborate and flexible motor program needed for retention and transfer of skills learnt. And thirdly, for providing additional insights into potential neural mechanisms – α and β oscillations – involved in processing information and how they might impact on motor skill acquisition and lead to more effectual skill retention and transfer. As this investigation incorporated a simple motor task, future research should incorporate a motor skill of greater complexity.