1. Background
Poor flexibility has been suggested to affect a person’s functional movement capacity with several authors noting that restricted hip extension is associated with poor neuromuscular efficiency and distorted movements patterns (1-3). The manifestation of restricted musculotendinous tissue has been proposed to be related to specific activity patterns, including certain sedentary positions. For example, restricted hip flexor length has been observed in both sedentary and active populations and is commonly considered to be related to excessive amounts of sitting, repetitive uniplanar movements, and/or improper movement techniques (1, 2).
Tight hip flexors can create altered reciprocal inhibition which modifies the agonist-antagonist relationship at the hip joint. A tight or restricted muscle can alter the length-tension relationships surrounding a joint and lead to altered muscle recruitment patterns (2). Shortened, overactive hip flexors can cause a decrease in neural drive to its functional agonist (gluteus maximus) during hip extension (4, 5). When the gluteus maximus is underactive, there is a greater reliance on the synergist muscle (hamstrings) to move the body through hip extension, a syndrome termed synergistic dominance (2, 4, 6). It has been proposed that synergistic dominance of the hamstrings leads to arthrokinetic dysfunction during sprinting and jumping movements, thus increasing the risk of hamstring injury (2, 7-9).
Functional movements are often used to assess arthrokinetic dysfunction to identify altered muscle recruitment patterns and muscular imbalances (2, 10). The squat, lunge, and step-up are common functional movements used to observe human movement impairments that may lead to potential risk for injury (2, 11-13). When a muscular imbalance is identified, specific corrective exercises can be prescribed to enhance one’s functionality and decrease risk of future injuries (2, 14-16).
2. Objectives
To the author’s knowledge, only one study has observed the effects of restricted hip extension on muscle activity during functional movement patterns (a bilateral air squat) (17). Additionally, it has been noted that males and females adopt different strategies to complete movements for a variety of reasons and that females have higher rates of lower extremity injury than males (18, 19). To better understand the effects of restricted hip extension on muscle activity in healthy females during functional movements, further investigation is required. The purpose of the study was to compare surface electromyography (sEMG) in the rectus femoris (RF), gluteus maximus (GM), biceps femoris (BF), and semitendinosus (ST) muscles and GM:BF co-activation ratio during the over-head squat, in-line lunge, and step-up between healthy females with and without hip flexor tightness. It was hypothesized that females without hip flexor tightness will have higher peak muscle activation of the gluteus maximus compared to those with hip flexor tightness.
3. Methods
3.1. Subjects
Twenty-three apparently healthy females (age: 22.00 ± 2.62 years; height: 162.84 ± 4.98 cm; body mass: 70.47 ± 14.84 kg; BMI: 26.48 ± 4.91) were assigned either to a control (n = 12) or experimental (n = 11) group determined from the modified Thomas Test. All participants were free of any lower body musculoskeletal injury within the past three months. After the participants were informed of the benefits and possible risks of the protocol, all participants completed an informed consent and PAR-Q+ pre-health screening. The Institutional Review Board at Middle Tennessee State University approved this study prior to data collection (ID #: 21-2124 4i).
The modified Thomas Test was used to assess hip flexor length because of its high inter-rater reliability (17, 20-22). A digital inclinometer (Model #12-1057, Fabrication Enterprise Inc. – Baseline Evaluation Instruments, White Plains, New York) was used to measure hip extension ROM during the modified Thomas Test. Inclinometer values greater than 0° (+) indicate that the thigh was positioned above parallel and relatively flexed. Inclinometer values below 0° (-) indicate that the thigh was below parallel and relatively extended (22). Inclusion criteria for the normal group was defined as hip extension ROM greater than 15° below parallel. Inclusion criteria for the experimental (tight hip flexor) group was defined as hip extension greater than 0° above parallel (17). The tightest leg was the experimental leg observed during the study for individuals with tight hip flexors, whereas the most flexible leg was the control leg observed for individuals without tight hip flexors in the study.
3.2. Procedures
Participants were required to attend a single session at the university muscle physiology laboratory. Upon attending the session, participants completed the informed consent and pre-health screening questionnaire (PAR-Q+) and were screened for inclusion criteria. If inclusion criteria were met into either group, participant’s age, height, and body were measured and recorded. Height was measured to the nearest 0.1 cm using a stadiometer (SECA Corporation, Model 222, Germany) and body mass was assessed using a digital scale (Tanita Worldwide, Model BF 522, Arlington Heights, Illinois) to the nearest 0.1 kg.
Muscle activity and kinematic data were measured using the Trigno wireless electromyographic (EMG) system (Delsys; Natick, MA). The system contained Trigno Flex EMG sensors that are placed directly on the skin surface over the mid-belly of indicated muscles. Prior to placing EMG sensors, hair was shaved with a safety razor when appropriate, exfoliated with Redux paste, and cleaned with isopropyl alcohol to reduce signal impedance. The underside of the sensors was attached to the skin with double-sided adhesive tape and then the outside of the sensor was further secured with adhesive stretch tape. Location and procedures for placement of sensors on the RF, GM, BF, and ST muscles was implemented in accordance with the SENIAM project guidelines (23).
Kinematic data (angular movement) was measured using wireless goniometers (Biometrics, Newport, UK) that were connected to Trigno Goniometer Adapters both of which were fixated to the skin in the same fashion described for the Trigno Flex sensors. The primary purpose of the kinematic data was to identify ascending and descending phases of movements. For knee joint angle, the proximal arm was aligned along the femur to the greater trochanter and the distal arm was aligned to the lateral tibia in line with lateral malleolus. All muscle activity and kinematic data was integrated directly into the EMGworks software via wireless adapters provided by the manufacture to ensure proper timing during recording. An external trigger device (Delsys, Natick, MA) was used to initiate and cease data collection. Prior to performing functional movements, participants performed three trials of maximal voluntary isometric contractions (MVIC). For each MVIC movement, participants were positioned to generate maximal force (24). Participants performed one practice trial, followed by three MVIC trials for each movement. Participants were instructed to hold the MVICs for 5 seconds and allotted 60 second rest between each trial. The highest peak MVIC per muscle was used for normalization of muscle activity during the functional movements.
3.3. Functional Movements
Prior to testing, participants were permitted to practice each functional movement until they performed each movement in a controlled manner, followed by a 5-minute warm-up on a stationary cycle ergometer. For each functional movement, participants were instructed to perform at least 3 repetitions with a 1- minute rest period between each repetition to prevent fatigue. A digital metronome was set 60 beats per minute for each functional movement and participants were asked to perform a three second eccentric phase and a two second concentric phase for overhead squat and in-line lunge and a two second concentric phase for the single leg step up. These three movements were included because they are integral parts of movement screening as described by the National Academy of Sports Medicine (NASM) movement assessment (2) and the Functional Movement Screen (FMSTM) (11). These are common screening tools used by practitioners to assess patients and client’s movement abilities and were selected because the hip musculature plays an integral role in completing these three tests.
3.3.1. Over-head Squat
The over-head squat was performed in a manner originally described by the National Academy of Sports Medicine (NASM) movement assessment (2). The participants were instructed to stand with their feet shoulder-width apart and pointed straight ahead on a stable surface. Participants then raised their arms overhead with elbows fully extended and were instructed to squat to roughly the height of a chair seat and return to a starting position.
3.3.2. In-line Lunge
The in-line lunge was performed in a similar manner originally described by the FMSTM (11). A certified athletic trainer attained the participants’ tibia length by measuring from the floor to the tibial tuberosity. The participant was then asked to place the end of their heel on a tape measure taped to the floor. The previous tibial measurement was then applied from the end of the toes of the foot on the floor and a mark was made. Finally, the participants were instructed to lower the back knee enough to touch the ground surface behind the heel of the front foot, while maintaining an upright posture, and then return to the starting position. The participants were informed to perform the in-lunge in a slow controlled fashion with both toes pointing forward and feet remaining flat. Participants were instructed to keep their hands on their hips.
3.3.3. Forward Step-up
Participants were asked to step-up onto a step (20 cm) with their tested foot, with their opposite foot trailing until both feet were firmly planted on top of the step. The participants were instructed to start the test directly in front of the step while keep their hands on their waist and knees straight. The non-tested leg was positioned over the floor adjacent to the step with the knee extended, while the tested leg was used to step-up until both feet were planted on top of the step.
3.4. Data Processing
All EMG data was normalized to maximal voluntary isometric contraction (MVIC) data collected for each participant to represent muscle activation of each muscle as a percent of peak muscle activity. Surface EMG data were processed using a Nyquist resampling equation at 1000 Hz, filtered with a Butterworth band-pass filter at 20 Hz and 450 Hz and a root-mean-square algorithm with a 200 ms window was then applied to the filtered data. Goniometer data was to mark and differentiate directional phases during the overhead squat and in-line lunge. The ascending phase of the forward step-up was the only phase observed because the purpose was to simulate the concentric nature of stepping up stairs. All data processing was performed using EMGworks analysis software (Delsys, Model SC-S08-4.5.3, Natick, MA) and exported to Microsoft excel (2016). The gluteus maximus: Biceps femoris co-contraction ratio was calculated by dividing the mean gluteus maximus activity by the mean biceps femoris activity (gluteus maximus: Biceps femoris), as described by Mills et al. (17). A gluteus maximus: Biceps femoris co-activation ratio of 1.0 indicates balanced muscular activation, whereas a ratio less than one 1.0 indicates greater activation of the biceps femoris relative to the gluteus maximus.
3.5. Data Analyses
The IBM© SPSS© Statistics (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp) was used for the statistical analysis. Descriptive statistics was provided for each participant and be expressed in means + standard deviations. Independent samples t-tests were conducted to compare participants with hip flexor tightness (n = 12) and without hip flexor tightness (n = 11) RF, BF, ST and GM mean muscle activity and GM:BF co-activation ratio during the overhead squat and in-line lunge, and forward step-up. Mean muscle activation was analyzed during the ascending and descending phases for the over-head squat and in-line lunge, whereas the only the ascending phase was observed during the forward step-up. Effect sizes were calculated using Hedges’ g. The alpha level was set at .004 using the Bonferroni correction for all statistical procedures.
4. Results
Results of the independent samples t-test comparing mean muscle activity of the muscles used in the study (RF, BF, ST, and GM) and GM:BF co-activation ratio during the ascending and descending phase of the overhead squat are displayed in Table 1.
| Variables | Non-Tight Hip Flexors | Tight Hip Flexors | t | P-Value | Mean Difference | 95% CI | Hedges’ g |
|---|---|---|---|---|---|---|---|
| DSC RF | 0.53 ± 0.40 | 0.51 ± 0.23 | 0.21 | 0.86 | 0.03 | [-0.26, 0.31] | 0.83 |
| DSC BF | 0.13 ± 0.08 | 0.27 ± 0.33 | 1.42 | 0.184 | 0.22 | [-0.56, 0.87] | 0.58 |
| DSC ST | 0.06 ± 0.09 | 0.09 ± 0.02 | 0.28 | 0.79 | 0.01 | [-0.07, 0.05] | 0.59 |
| DSC GM | 0.09 ± 0.03 | 0.09 ± 0.05 | 0.57 | 0.58 | 0.02 | [-0.04, 0.07] | 0.22 |
| DSC GM:BF Co-A | 0.75 ± 0.50 | 0.56 ± 0.40 | 1.01 | 0.32 | 0.19 | [-0.20, 0.58] | 0.41 |
| ASC RF | 0.67 ± 0.54 | 0.50 ± 0.24 | 0.97 | 0.35 | 0.17 | [-0.20, 0.53] | 0.42 |
| ASC BF | 0.23 ± 0.19 | 0.33 ± 0.31 | 1.04 | 0.31 | 0.14 | [-0.41, 0.13] | 0.37 |
| ASC ST | 0.12 ± 0.17 | 0.24 ± 0.36 | 1.13 | 0.26 | 0.19 | [-0.55, 0.17] | 0.43 |
| ASC GM | 0.13 ± 0.08 | 0.15 ± 0.06 | 0.58 | 0.57 | 0.02 | [-0.08, 0.05] | 0.23 |
| ASC GM:BF Co-A | 0.70 ± 0.36 | 0.73 ± 0.47 | 0.18 | 0.86 | 0.03 | [-0.39, 0.32] | 0.07 |
Abbreviations: DSC, descending; ASC, ascending; RF, rectus femoris; BF, biceps femoris; ST, semitendinosus; GM, gluteus maximus; Co-A, co-activation.
a Values are expressed as mean ± SD.
Mean muscle activity in the RF, BF, ST, and GM were not significantly different in female participants with and without hip flexor tightness during the descending and ascending phases of the overhead squat. Although, mean BF muscle activity in participants with tight hip flexors (M = 0.27, SD = 0.33) was higher compared to participants without tight hip flexors (M = 0.13, SD = 0.08) during the descending phase of the overhead squat, but was not statistically significant (P = 0.18). Similar results were seen during the ascending phase of the overhead squat where individuals with tight hip flexors displayed higher mean BF (M = 0.33, SD = 0.31) and higher mean ST (M = 0.24, SD = 0.36) compared to mean BF activity (M = 0.23, SD = 0.19) and mean ST activity (M = 0.12, SD = 0.17) in those without hip flexor tightness. However, there was no statistical difference between mean BF (P = 0.31) and mean ST (P = 0.26) activity in those with and without hip flexor tightness during the ascending phase of the overhead squat.
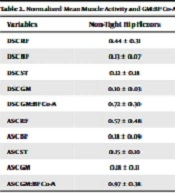
Results of the independent samples t-test comparing mean muscle activity of the RF, BF, ST, and GM and GM:BF co-activation ratio during the ascending and descending phase of the in-line lunge are displayed in Table 2. Mean muscle activity in the RF, BF, ST, and GM were not significantly different in female participants with and without hip flexor tightness during the descending and ascending phases of the in-line lunge. During the ascending phase of the in-line lunge, individuals with tight flexors also displayed higher mean BF activity (M = 0.28, SD = 0.21) compared to individuals without tight hip flexors (M = 0.18, SD = 0.09), but was not statistically significant (P = 0.16).
| Variables | Non-Tight Hip Flexors | Tight Hip Flexors | t | P-Value | Mean Difference | 95% CI | Hedges’ g |
|---|---|---|---|---|---|---|---|
| DSC RF | 0.44 ± 0.31 | 0.44 ± 0.18 | 0.04 | 0.97 | 0.004 | [-0.23, 0.22] | 0.02 |
| DSC BF | 0.13 ± 0.07 | 0.27 ± 0.28 | 1.52 | 0.16 | 0.14 | [-0.24, 0.04] | 0.69 |
| DSC ST | 0.12 ± 0.18 | 0.11 ± 0.08 | 0.26 | 0.80 | 0.01 | [-013, 0.21] | 0.10 |
| DSC GM | 0.10 ± 0.03 | 0.09 ± 0.03 | 0.35 | 0.73 | 0.008 | [-0.04, 0.06] | 0.14 |
| DSC GM:BF Co-A | 0.72 ± 0.30 | 0.63 ± 0.56 | 0.49 | 0.63 | 0.09 | [-0.29, 047] | 0.20 |
| ASC RF | 0.57 ± 0.48 | 0.46 ± 0.17 | 0.71 | 0.49 | 0.10 | [-0.21, 0.42] | 0.28 |
| ASC BF | 0.18 ± 0.09 | 0.28 ± 0.21 | 1.43 | 0.16 | 0.10 | [-0.25, 0.08] | 0.59 |
| ASC ST | 0.15 ± 0.10 | 0.14 ± 0.10 | 0.09 | 0.93 | 0.005 | [-0.10, 0.05] | 0.04 |
| ASC GM | 0.18 ± 0.11 | 0.18 ± 0.09 | 0.24 | 0.81 | 0.01 | [-0.09, 0.08] | 0.09 |
| ASC GM:BF Co-A | 0.97 ± 0.38 | 0.99 ± 0.41 | 0.06 | 0.95 | 0.02 | [-0.55, 0.58] | 0.04 |
Abbreviations: DSC, descending; ASC, ascending, RF, rectus femoris; BF, biceps femoris; ST, semitendinosus; GM, gluteus maximus; Co-A, co-activation.
Results of the independent samples t-test comparing for mean muscle activity of the RF, BF, ST, and GM and GM:BF co-activation ratio in those with and without hip flexor tightness during the ascending of the in-line lunge are displayed in Table 3. Mean muscle activity in the RF, BF, ST, and GM were not significantly different in female participants with and without hip flexor tightness during the descending and ascending phases of the forward step-up. During the ascending phase of the forward step-up, individuals with hip flexor tightness displayed higher mean BF (M = 0.30, SD = 0.41) activity and ST (M = 0.27, SD = 0.43) activity compared to mean BF (M = 0.10, SD = 0.06) and ST (M = 0.14, SD = 0.43) activity in those without hip flexor tightness. However, there was no statistical difference between mean BF (P = 0.13) and mean ST (P = 0.41) activity in those with and without hip flexor tightness during the ascending phase of the forward step-up.
| Variables | Non-Tight Hip Flexors | Tight Hip Flexors | t | P-Value | Mean Difference | 95% CI | Hedges’ g |
|---|---|---|---|---|---|---|---|
| ASC RF | 0.26 ± 0.20 | 0.25 ± 0.12 | 0.10 | 0.92 | 0.007 | [-0.13, 0.15] | 0.04 |
| ASC BF | 0.10 ± 0.06 | 0.30 ± 0.23 | 1.73 | 0.11 | 0.12 | [-0.48, 0.07] | 0.73 |
| ASC ST | 0.14 ± 0.26 | 0.17 ± 0.15 | 0.37 | 0.71 | 0.03 | [-0.43, 0.18] | 0.15 |
| ASC GM | 0.10 ± 0.07 | 0.10 ± 0.04 | 0.035 | 0.97 | <0.001 | [-0.05, 0.51] | 0.01 |
| ASC GM:BF Co-A | 0.96 ± 0.41 | 0.80 ± 0.61 | 0.89 | 0.39 | 0.18 | [-0.25, 0.64] | 0.35 |
Abbreviations: DSC, descending; ASC, ascending; RF, rectus femoris; BF, biceps femoris; ST, semitendinosus; GM, gluteus maximus; Co-A, co-activation.
4. Discussion and Conclusions
The purpose of this study was to compare mean muscle activity of the RF, BF, ST, GM and GM:BF co-activation ratio between females with and without hip flexor tightness during the overhead squat, in-line lunge, and forward step-up. Contrary to our hypothesis, no statistical differences were found in mean muscle activation of the RF, BF, ST, and GM and GM:BF co-activation ratio. Mean GM activity was similar in those with and without hip flexor tightness during all three functional movements. However, individuals with hip flexor tightness displayed higher mean BF activation during the overhead squat, in-line lunge, and forward step-up, indicating an increased utilization of the hamstrings musculature to complete functional movements in those who have restricted hip extension.
It has been speculated that tight hip flexors may cause reliance on secondary hip extensors, potentially provoking greater stress on the hamstrings (17, 25-27). Over activation of the hamstrings has been linked to those with lower extremities injuries (28-30). One study found that participants with osteoarthritis displayed increased semitendinosus (SMT) and BF muscle activity during mid-stance, late stance and early swing phase of a gait cycle (30). Similar results were seen in those with patellofemoral pain syndrome (PPS) where those with PPS generated greater BF activity during walking gait (28). In addition, Emami et al. (29) observed greater mean BF activity in those with hamstring injury compared to those without during a prone hip extension test. A study by Daly et al. (25) compared BF:GM muscle activation in male athletes with and without a previous hamstring injury during running gait and found that individuals with a previous hamstring injury had greater BF:GM muscle activation compared to those without a previous hamstring injury; indicating greater BF muscle activity compared to the GM. In the current study, mean BF activity was higher in those with hip flexor tightness during the over-head squat, in-line lunge, and forward step-up, but was not statistically significant. Therefore, more investigation on hip flexor tightness and hamstring activity is inquired, as previous literature has shown tight hip flexors may cause changes in the neuromuscular control of the lumbopelvic hip complex, specifically the BF.
While not statistically significant, the present study did see a rather large increase in BF activity in those who had restricted hip extension during all functional movements. The overhead squat saw a 70% higher muscle activity during the descending phase (Hedges’ g = 0.58) and 35% higher muscle activity during the ascending phase (Hedges g = 0.37). The inline lunge saw 70% greater muscle activity during the descending phase (Hedges’ g = 0.69) and 43% greater muscle activity during the ascending phase (Hedges’ g = 0.59). The forward step-up saw an increase of 100% during the ascending phase (Hedges’ g = 0.73). These changes occurred in stark contrast to changes in GM activity, which were no greater than 14% during any movement. While the lack of statistical significance makes it impossible to draw any firm conclusions surrounding these findings, it would appear to suggest that those with tight hip flexors display increased hamstring muscle activity during functional movements. However, the lack of statistically significant findings contradicts the findings of the one other study which has assessed muscle activity during functional movement patterns (17).
Mills et al. (17) investigated the effect of hip flexor tightness on muscle activity during a bilateral air squat in female soccer players. Contrary to the current study, they found a statistically significant decrease in gluteus maximus activity in those with tight hip flexors compared to those without. Additionally, they saw a non-significant reduction in BF muscle activity which resulted in a decrease in GM:BF ratio in those with tight hip flexors, thus indicating an increased reliance on the BF during the functional bilateral squat. This has led the current authors to postulate that there are three potential reasons that the current study lacked a statistically significant difference in hamstrings activity during the three tested functional movements.
In their study Mills et al. (17) examined collegiate soccer females, an active and likely resistance trained population. In contrast, the current study did not consider activity level in the sample resulting in a more diverse group of participants, many of whom were likely not trained. It is well established that individuals who are resistance trained and physically active have greater motor unit activation and neuromuscular control compared to their untrained counterparts (31-33). However, this study included individuals who were both active and non-active, potentially influencing mean EMG muscle activity during functional movements more than hip flexor tightness. As the participants in the Mills et al. (17) study were trained, they are likely capable of recruiting a greater total number of motor units at a lower threshold. This could potentially result in a greater differential between those who have and those who do not have hip flexor tightness, creating the statistically significant reduction of muscle activity of the GM in those that have hip flexor tightness in an active population.
A second potential reason for not seeing statistical significance may be more mechanistic in nature. Proper form and greater neuromuscular activation is more likely seen in individuals who are physically active and resistance trained, regardless of hip flexor tightness. Muscle imbalances caused by hip flexor tightness may affect a physically active and inactive population differently and this is the primary difference between Mills et al. (17) study and the current study. More specifically, the mechanism that incites a muscular imbalance in a specific population may cause distinct changes in muscular activation. This suggests that muscular imbalances caused by hip flexor tightness may affect a physically active and inactive population differently due to different causational mechanisms that lead to hip flexor tightness. The complexity of the musculoskeletal system makes the exact cause of muscle imbalances difficult to identify, but possible mechanisms include improper habitual patterns, chronic repetition of a movement, and altered movement due to previous injury (2, 4, 10). The mechanism that causes hip flexor tightness may impact the musculoskeletal compensations and therefore influence muscular activity differently. Mills et al. (17) used female soccer players who were thought to obtain hip flexor tightness from chronic repetitive hip flexion. In the current study, both active and inactive participants who partook in the study may have obtained hip flexor tightness from other types of mechanisms. Therefore, the mechanism itself that caused hip flexor tightness may influence muscle activity during functional movements.
A final potential reason the current study did not see a significant difference in muscle activity is low absolute loading. It has been well established that as external load on the body or a muscle increase, muscle activity also increases (19). The functional movements tested in this study did not apply any external loading above the participants bodyweight. Additionally, these tests did not require the participant to perform a gait cycle. Instead, these tests were performed in a controlled manner that minimized ground reaction force. Studies have seen that a task such as gait produces up to 50% greater ground reaction forces than squatting (34). This means that to complete the tasks in this study, there is minimal ground reaction force the body must dissipate. As a result, it may not have necessitated a high level of recruitment from synergist muscles, like the BF, to see statistically significant differences.
The main limitation of this study was low sample size and unequal groups, increasing the likelihood of type II error. Another limitation to this study was the calculation of the GM:H ratio only considered the BF (17) which may not fully represent the function of the hamstring muscle group during hip extension. An additional limitation is that activity level was not controlled for, which may have been the reason that there was no difference between groups. The findings of this study call for further investigation on the effect of hip flexor tightness of muscle activity during functional movements. Future studies should investigate muscle activation in those with and without hip flexor tightness under different external loads while controlling for activity level. Future research should also aim to confirm the effect of hip flexor tightness on muscle activity and its relationship to hamstring injury and further consider the potential role that the cause of the hip flexor tightness may play in explaining altered muscle activity in the lumbo-pelvic hip complex.
The results of this study did not yield any statistically significant differences in muscle activity between those with and without hip flexor tightness. However, those with hip flexor tightness did see a moderate to large increase in BF activity during all functional movements as evidenced by the effect sizes. This increased reliance on the hamstrings could potentially place people at risk for injury. The lack of statistically significant findings contradicts previous research (17) and suggest the need for further investigation in this area.