1. Background
Sleep is vital to human health and a physiological necessity that significantly affects the quality of life and well-being (1-3). Pregnant women need healthy sleep to develop the fetus and gain the necessary energy to give birth (1, 2). Sleep is also essential for metabolism, immunity, and hormone function (3). Changes that occur to meet the fetus’s needs during pregnancy and prepare the woman’s body for childbirth affect sleep routine and duration, making it difficult to fall asleep and maintain it (2, 4). Even women, who do not suffer from sleep problems, can experience profound sleep problems during pregnancy (4).
It is reported that poor sleep quality leads to interventions during labor, such as forceps, vacuum, episiotomy, or induced labor, more fatigue during labor, the development of complications such as anxiety, decreased tolerance to pain, deterioration of blood pressure, and hypertension. In women with poor sleep quality, it is an increased probability of cesarean section, low birth weight, premature birth or stillbirth, prolonged vaginal delivery, and depression during pregnancy and postpartum, negatively affecting their quality of life (2, 5-11).
It is essential to know pregnant women’s sleep characteristics and problems due to the adverse effects of poor sleep quality on birth, mother, and newborns. Although sleep disorders are common during pregnancy, it is observed that nurses do not adequately question the sleep quality of pregnant women while providing antenatal care during routine hospital control. Knowing the sleep quality of the mother during pregnancy and making effective nursing and midwifery interventions for women suffering from sleep problems can allow women to have a healthier pregnancy, birth, and postpartum process. This study was planned to determine the effect of sleep quality on the duration of delivery and labor pain. The data is expected to provide clues for nursing and midwifery approaches to solving sleep problems during pregnancy.
2. Objectives
This study was planned to determine the effect of sleep quality on the duration of delivery and labor pain. The data is expected to provide clues for nursing and midwifery approaches to solving sleep problems during pregnancy.
2.1. Research Questions
- Does the sleep quality of pregnant women affect the duration of delivery and labor pain?
- Does the daytime sleepiness of pregnant women affect the duration of delivery and labor pain?
3. Methods
3.1. Study Design and Setting
This study is descriptive based on repeated measurements.. It was conducted in an obstetrics and gynecology training and research hospital affiliated with the Ministry of Health in Turkey.
3.2. Participants
Within the data collection process, 350 pregnant women applied to the maternity hospital, and only 160 were determined to have a gestational age of ≥ 38 weeks. The study population comprised 160 pregnant women who were expected to give vaginal delivery and met the inclusion criteria. An expert statistician calculated the sample size according to the acceptable error and confidence level. The power analysis is performed by the G*Power (3.1.9.2) software, at the acceptable error of 5% and confidence level of 95%, the sample size was determined to be 114 pregnant women. Thus, 114 pregnant women who met the inclusion criteria were tried to be accessed during the data collection period.
A total of 118 pregnant women were interviewed for data collection in the allocated time. We excluded 14 pregnant women who gave birth via cesarean section before the onset of normal birth and 29 who had cesarean delivery because of cephalopelvic disproportion. Thus, the sample consisted of 75 pregnant women.
Inclusion criteria for the pregnant women were as follows: (1) gestational age of > 38 weeks; (2) no lack of position and presentation anomalies; (3) no risk of pregnancy (diabetes, hypertension, pre-eclampsia, etc.); (4) planning to give birth at the hospital where they were followed up; (5) not undergoing epidural anesthesia during labor; (6) planning to have a vaginal delivery; (7) not receiving analgesics during labor; and (8) being capable of verbal communication. Exclusion criteria were (1) giving birth by cesarean section before the onset of normal birth; and (2) having cesarean delivery because of cephalopelvic mismatch.
The dependent variables were sleep quality scores (Pittsburgh Sleep Quality Index and Epworth Sleepiness Scale) and pain scores. Independent variables included socio-demographic characteristics (age, education, working status, tea and coffee consumption, smoking, drug use, sleep-related habits, and exercise), obstetric history (pregnancy/birth/abortion/curettage/number of children, gestational week, disease, and pregnancy problems), and delivery information (labor stages I, II, or III and the total duration of delivery).
3.3. Data Collection Tools
A researcher-made form for personal information and socio-demographic characteristics, Pittsburgh Sleep Quality Index, Epworth Sleepiness Scale, and Visual Analog Scale-Pain were administered to all pregnant women.
3.3.1. Personal Information Form
This form was used to assess the participants’ demographic, obstetric, pregnancy, and delivery characteristics.
3.3.2. Pittsburgh Sleep Quality Index
The Pittsburgh Sleep Quality Index (PSQI) was developed by Buysse et al. (12) and adapted into Turkish by Agargun et al. (13) The scale consists of 24 items and seven subscales, including subjective sleep quality, sleep duration, sleep latency, sleep disturbances, habitual sleep efficiency, use of sleeping medication, and daytime dysfunction. The 19th question is not taken into account in the scoring of the scale. The last five questions are used only for clinical information and are not added to the scale score. The remaining 18 questions of the scale are scored between 0 and 3 according to symptom frequency. The total score of the index ranges between 0 and 21 points. High scores indicate poor sleep quality. A total global sleep quality score of < 5 is evaluated as good sleep quality, and a score of ≥ 5 is evaluated as poor sleep quality. It is a valid and reliable scale (Cronbach’s alpha coefficient of 0.80) (12, 13). In this study, Cronbach’s alpha reliability coefficient was 0.76.
Epworth Sleepiness Scale: This scale was developed by Johns (14) and adapted into Turkish by Agargun et al. (15) It questions eight states that induce sleep. Each item on the scale is rated 0 - 3, and the total score ranges from 0 - 24 points. A score of 1-6 is evaluated as good sleeping, a score of 10 - 16 as sleepy, and a score of 16 or more as severely drowsy. It is a valid and reliable scale (Cronbach’s alpha coefficient of 0.80) (14, 15). In this study, Cronbach’s alpha reliability coefficient was determined to be 0.64.
3.3.3. Visual Analog Scale (VAS)
This scale is used to assess pain level, and some digitized values cannot be measured numerically. The patients are asked to mark any point that fits their situation on a 10-cm line, including no pain on one edge and worst pain on the other edge. The distance between 0 (no pain) and the marked point is measured using a ruler. High scores indicate severe pain (16, 17). If the evaluation is made on the same scale, the patient may make evaluations based on previous pain scores; therefore, a new scale should be used every time in repeated assessments (18).
3.4. Data Collection
The study was conducted in two stages.
- The women were interviewed face-to-face in an appropriate room, and the Personal Information Form and Sleep Quality Scales were completed. Then, the women were given a card including the researcher’s contact details and asked to inform the researcher when the birth would start.
- When these women came for labor, they were followed during labor, and their labor information was obtained. In order to evaluate the relationship between sleep and labor pain, the level of pain was determined using the Visual Analog Scale-Pain three times (when the cervical dilatation was 1 - 3 cm, when it was 4 - 10 cm, and after the birth of the placenta). The mother’s statements were taken at intervals without pain. A new scale was used every time.
3.5. Data Analysis
SPSS version 15.0 (IBM Corporation, Armonk, NY, USA) software was used to analyze the data. Medians, arithmetic means [standard deviation (SD)], frequency, percentages, minimum, and maximum were used to evaluate the data. In comparing the data, the Fisher’s exact test, Kruskal-Wallis test, and Mann-Whitney U test were used. Cronbach’s alpha reliability coefficient was used to assess the reliability of the scales. The statistical significance was set at P < 0.05.
3.6. Research Ethics
The study was approved by the Ethics Committee of the Medical Faculty (No. 40223-B-06). The pregnant women were informed about the aim and benefits of the study and their roles. Written and verbal consent was obtained from the pregnant women. They were assured that their information would be kept confidential and only used for scientific purposes. No names were entered on the data collection form to maintain their privacy.
4. Results
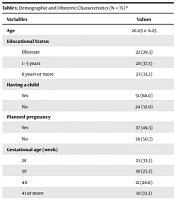
The sample consisted of 75 pregnant women. The participants’ ages ranged from 16 - 42 years, and their mean age was 26.05 (± 6.25). Also, 37.3% of the pregnant women went to school for 1 - 5 years, 68% had a child, 49.3% had planned their pregnancy, 33.3% were in the 38th gestational week, and 73.3% were multigravida (Table 1).
| Variables | Values |
|---|---|
| Age | 26.05 ± 6.25 |
| Educational status | |
| Illiterate | 22 (29.3) |
| 1 - 5 years | 28 (37.3) |
| 6 years or more | 25 (33.3) |
| Having a child | |
| Yes | 51 (68.0) |
| No | 24 (32.0) |
| Planned pregnancy | |
| Yes | 37 (49.3) |
| No | 38 (50.7) |
| Gestational age (week) | |
| 38 | 25 (33.3) |
| 39 | 19 (25.3) |
| 40 | 21 (28.0) |
| 41 or more | 10 (13.3) |
| Gravida | |
| Primigravida | 20 (26.7) |
| Multigravida | 55 (73.3) |
a Values are expressed as mean ± SD or No. (%).
The problems often experienced by pregnant women were frequent urination (74.7%), back/waist/groin pain (72%), and leg pain (70.7%). When problems affecting the quality of sleep during pregnancy were examined, there was a statistically significant difference between poor sleep quality and leg pain/cramps (P = 0.04), frequent urination (P = 0.02), and mental distress/tension problems during pregnancy (P = 0.02).
There was no statistical difference between the components and total scores of Pittsburgh Sleep Quality Index and age, gestational week, gravida status, and having a child. Besides, 89.3% of pregnant women had poor sleep quality. They slept for a mean of 6.57 (± 2.02) (min - max 3 - 10) hours, 90.6% were awake, and 41.3% had bad dreams at night.
When the sleep quality of pregnant women was compared with characteristics related to the stages of labor, the duration of the second stage of labor was statistically longer in women who had good sleep quality (P < 0.001) (Table 2).
| Duration of Labor Stages (min) | Pittsburgh Sleep Quality Index | MW-U | P | Epworth Sleepiness Scale | MW-U | P | ||
|---|---|---|---|---|---|---|---|---|
| Good Sleep Quality (n = 8) | Poor Sleep Quality (n = 67) | Good Sleeping (< 10 Points) (n = 68) | Sleepy (≥ 10 Points) (n = 7) | |||||
| First stage | 670.00 ± 413.49 | 843.58 ± 521.75 | 215.50 | 0.36 | 825.17 ± 525.19 | 824.00 ± 387.06 | 215.50 | 0.68 |
| Second stage | 15.00 ± 7.55 | 7.66 ± 4.42 | 100.00 | 0.00 b | 9.89 ± 13.94 | 9.71 ± 6.18 | 206.50 | 0.55 |
| Third stage | 10.12 ± 2.94 | 14.50 ± 8.67 | 181.50 | 0.10 | 14.38 ± 8.68 | 10.71 ± 1.88 | 182.50 | 0.26 |
| Total duration of labor | 695.12 ± 415.10 | 866.83 ± 521.68 | 216.00 | 0.37 | 848.94 ± 525.03 | 844.42 ± 389.34 | 216.00 | 0.68 |
Abbreviation: MW-U, Mann-Whitney-U.
a Values are expressed as mean ± SD.
b P < 0.05.
According to the scores of the Epworth Sleepiness Scale, 90.7% of the pregnant women were good sleepers (< 10 points), and 9.3% had daytime sleepiness (≥ 10 points). When the Epworth Sleepiness Scale scores of the pregnant women were compared with the characteristics of the stages, no statistically significant difference was found (Table 2).
According to the Pittsburgh Sleep Quality Index and Epworth Sleepiness Scale, the pain scores of pregnant women who had good and bad sleep quality in stages of labor were examined, showing no statistically significant difference (Table 3).
| Pain Score in Stages of Labor | Pittsburgh Sleep Quality Index | MW-U | P | Epworth Sleepiness Scale | MW-U | P | ||
|---|---|---|---|---|---|---|---|---|
| Good Sleep Quality (n = 8) | Poor Sleep Quality (n = 67) | Good Sleeping (< 10 Points) (n = 68) | Sleepy (≥ 10 Points) (n = 7) | |||||
| The first stage | ||||||||
| Latent phase | 5.06 ± 3.59 | 3.84 ± 2.66 | 208.00 | 0.30 | 3.83 ± 2.68 | 5.28 ± 3.43 | 171.50 | 0.22 |
| Active phase | 8.75 ± 1.58 | 9.17 ± 1.57 | 216.00 | 0.28 | 9.12 ± 1.59 | 9.14 ± 1.46 | 235.50 | 0.95 |
| The third stage | ||||||||
| After the birth of the placenta | 3.68 ± 3.43 | 5.15 ± 3.28 | 194.50 | 0.20 | 4.97 ± 3.27 | 5.28 ± 3.86 | 233.50 | 0.93 |
Abbreviation: MW-U, Mann-Whitney-U
a Values are expressed as mean ± SD.
b P > 0.05.
5. Discussion
Changes that occur to meet the needs of the fetus and the preparation of the woman’s body for childbirth affect sleep routine and duration. They make it difficult to fall asleep and maintain sleep (2, 4). Poor sleep quality leads to prolonged labor, assisted delivery, more fatigue during labor, complications such as anxiety, decreased tolerance to pain, and hypertension. The probability of cesarean section, premature birth or stillbirth, prolonged duration of vaginal delivery, and low Apgar score increases in women with poor sleep quality (2, 5, 8-11).
Pregnancy can cause sleep disorders and exacerbate existing sleep problems (2). Lara-Carrasco et al. (19) determined that pregnant women reported lower sleep quality and more night awakenings than nonpregnant women. While Owusu et al. (8) found that 86.4% of pregnant women had poor sleep quality. Sut et al. (10) stated that 34.2% of women had poor sleep quality. Hung et al. (2) found that the prevalence of poor sleepers (PSQI score > 5) was 66% in the third trimester. Rezaei et al. (7) found that the mean sleep quality was 8.6 (± 2.8). Ozkan and Rathfisch (11) found that 60.7% of participants had poor sleep quality, with a score of 7.1 (± 3.6). In this study, 89.3% of women had poor sleep quality, with a PSQI score of 8.7 (± 3.5). These results are compatible with Owusu et al. (8) and Rezaei et al. (7) This study was conducted on women in the third trimester. Sleep quality is impaired mainly during the third trimester (5-8, 10, 11). This is the reason for the high rate of pregnant women with poor sleep quality.
During the first trimester of pregnancy, women tend to sleep during the day and have an increase in total sleep duration. Total sleep duration normalizes in the second trimester. In the last trimester, the pregnant women’s sleeping habits and sleep quality are impaired (10, 20, 21). Lee and Caughey (22) specified that most women awoke two or three times during the third trimester and slept for an average of 7.5 hours at night. Jamalzehi et al. (5) found that women in the 28 - 40 gestational weeks slept for an average of 8.7 (± 1.7) hours. Owusu et al. (8) found that sleep duration was 8.3 (± 2.2) hours, 16.8% of the women slept for less than six hours, and 25.9% slept for more than 10 hours. Facco et al. (23) found that the average sleep duration of women was lower in the third trimester. The present study determined that 90.6% of women in the third trimester woke up at least once during the night and slept for 6.5 hours per night. As can be seen from these results, when compared with the literature, the duration of sleep was low, and the rate of awakening at night was the same as the rate reported by Lee and Caughey (22).
Anatomical, physiological, and biochemical changes in pregnancy make it difficult to fall asleep, so sleep quality is impaired. In the last trimester, pressure exerted by the fetus on the diaphragm, fetal movements, pain, birth anxiety, back pain, leg cramps, restless leg syndrome, frequent urination, reflux, heartburn, and nausea affect the sleep habits of pregnant women (10, 20, 21). These problems experienced by pregnant women are reported to impair the quality of sleep (22, 24). Lee and Caughey (22) reported that the vast majority of women during the third trimester (65 - 80%) experienced frequent urination, back pain, and leg cramps. Jamalzehi et al. (5) found that common problems included frequent urination, hot flashes, digestive problems, nausea, vomiting, and shortness of breath, and the most frequent causes of waking up included hot flashes and frequent urination. In the present study, pregnant women often experienced problems such as frequent urination (74.7%), back/waist/groin pain (72%), and leg pain (70.7%). The results of the present study are compatible with the results of other studies. The sleep quality of pregnant women with leg pain/cramps and frequent urination problems was poor.
Bad dreams have been reported to be widely seen during pregnancy because of anxiety, sleep disorders, or change in hormone levels (25). Schredl et al. (26) reported that the frequency of disturbing dreams was higher in pregnant women. More than 11% of women had reported nightmares once a week or more (26). Nielsen and Paquette (27) found that 34% of pregnant women had frightening dreams and nightmares. In their study, Lara-Carrasco et al. (19) determined that 21% of pregnant women reported a nightmare incidence. In our study, 41.3% of women had bad dreams. These results are higher than the results reported in the literature.
The literature reports that sleep quality affects the labor (2, 28, 29). Lee and Gay (28) determined that sleep quality affects the duration of labor, and women with shorter sleep were 4.5 - 5.2 times more likely to have a cesarean section. Zafarghandi et al. (29) found that sleep quality could decrease the third stage of labor. In the present study, pregnant women with poor sleep quality had a significantly shorter second stage of labor (P < 0.001). This result was associated with a small sample size. Further studies should be conducted on this subject.
Poor sleep quality reduces the ability to recover and rest, leading to decreased energy and increased fatigue (30). Fatigue may influence the ability to endure labor pain and maternal pushing to deliver the fetus successfully during labor (23, 31). Lack of sleep reduces the pain threshold, whereas sleep and rest increase the pain threshold (32, 33). It was reported that sleep durations of < 5 hours were associated with increased pain. While sleep of fewer than 6 hours is associated with increased pain symptoms, it is seen that pain symptoms reduce in sleep durations of 7 - 8 or 8 - 9 hours (3). Bebee and Lee (30) determined a correlation between sleep five days before giving birth and pain and fatigue during labor. In this study, pregnant women’s sleep quality and sleepiness status during the day did not affect the pain score. The results of this study do not support the literature. The sleep scale used in this study examines the sleep quality during the last month, whereas Bebee and Lee (30) used wrist actigraphy monitors in their study. Different methods used for the measurements may have influenced the results. Further studies are suggested.
5.1. Limitations
Some participants were excluded from the study due to cesarean section before or after the onset of labor because of cephalopelvic mismatch. This study’s results cannot be generalized because it was performed in a single center with a limited sample.
5.2. Conclusions
Pregnant women with poor sleep quality had a significantly shorter second stage of labor. Sleep quality and sleepiness status during the day did not affect the pain. It was determined that pregnant women wake up frequently at night and have poor sleep quality. Leg pain/cramps and frequent urination negatively affected sleep quality.