1. Background
Congenital hypothyroidism (CH) is the most common inherited hormonal disease and one of the preventable causes of mental retardation (1); however, its diagnosis may be missed in infants due to the relative passage of maternal thyroid hormone through the placenta (2). The prevalence of CH has been reported to be 1: 3000 to 1: 4000 (3) and 1/446 live births in Iran (4).
The most important causes of transient CH are prematurity and thyroid-stimulating hormone (TSH) receptor-blocking antibodies due to maternal Hashimoto’s disease (3). Two studies have investigated the association between hypothyroidism in pregnant women and thyroid function in their fetuses (5, 6), suggesting that these babies should be screened for thyroid function (TSH and free thyroxine (fT4)) at 10 to 14 days after birth (6).
Triiodothyronine (T3) and thyroxin (T4) are produced at 12 weeks of gestation and TSH at 10-12 weeks. Since the fetal thyroid axis does not function independently until mid-pregnancy age, the transfer of T4 through the placenta is critical for fetal brain development, so untreated maternal hypothyroidism may lead to the poor growth of the fetal nervous system (7).
The factors affecting neurodevelopment include fetal age at the onset of treatment, the starting dose of levothyroxine, the severity of hypothyroidism, the levels of serum T4 in the first two years of life, and early diagnosis and treatment of the disease (8). In most studies performed before the onset of screening programs, an inverse relationship has been found between the age of diagnosis and the child’s intelligence quotient (IQ) (8). A study showed that IQ and motor development were normal in children who had normalized T4 levels after receiving a high initial dose of levothyroxine and undergoing careful monitoring and dose adjustment during the first two years of life (9). Moreover, another study demonstrated that levothyroxine therapy at the end of the first week of life would improve brain development only in extremely preterm newborns (< 28 weeks) (10). A new study in the Netherlands found no association between the time of treatment onset and developmental outcomes among children who received T4 from the ninth day of birth. However, some children with severe CH were at a greater risk for motor and cognitive problems (11).
Recently, a study on infants whose mothers had subclinical hypothyroidism showed a decrease in the brain growth index at 6 and 12 months of age but not after 24 months, when motor function and nerve development were normal (12). Only one study found that severe hypothyroidism in the first trimester was ineffective if thyroid function was normal at other trimesters (13).
Maternal hypothyroidism can be one of the risk factors for hypothyroidism in babies. So far, few studies have been conducted in this field. In a study in 2013, it was concluded that babies born to mothers with hypothyroidism had higher serum levels of TSH and were at a higher risk for the incidence of other thyroid problems (14). In another study in China, risk factors for CH were investigated in 2,771,661 infants, reporting that the family history of thyroid diseases was among the most important triggers; however, this study specifically did not mention if a history of maternal hypothyroidism during pregnancy could be a risk factor (15). In two retrospective studies on infants born to mothers with hypothyroidism, no significant association was found between maternal hypothyroidism and the risk of CH, but it was suggested that infants of these mothers should be screened routinely for thyroid function, but not on days 10 to 14 after birth (16, 17).
2. Objectives
There are conflicting results regarding the relationship between a history of hypothyroidism in pregnant mothers and CH in infants. Therefore, this study aimed to assess the relationship between maternal hypothyroidism and CH. Our results can help refine screening and follow-up programs for these babies, which are currently the same as those for babies born to otherwise healthy mothers.
3. Methods
3.1. Study Design and Setting
This cross-sectional descriptive-analytical study was conducted on all the neonates born in Fars province, Iran, from 2018 to 2020. This study was approved by the Ethics Committee of Shiraz University of Medical Sciences (Ethics code: IR.SUMS.MED.REC.1399.345).
3.2. Participants
Based on the survey conducted by Aminzadeh (4), considering the prevalence of 1:446 for CH in Iran, an error rate of 5%, and precision (d) of 10%, the sample size was calculated to be 175,100.
All infants born in Fars province, Iran, between April 2018 and September 2020, undergoing national health care and screening for congenital genetic, metabolic, and endocrine diseases, including hypothyroidism, whose information was registered in the non-communicable diseases unit of the health department of Shiraz University of Medical Sciences were included.
Exclusion criteria were incomplete or inaccessible information about babies or mothers and parental refusal to conduct subsequent screening and confirmatory tests or periodic follow-ups.
3.3. Diagnostic Criteria and Study Procedure
According to the national screening program, heel sampling was performed on a filter paper for all babies within 3-5 days after birth. Then TSH concentration was measured in heel blood samples using the filter paper as a primary screening test. In cases where the TSH level in the screening test and the serum confirmation test exceeded 20 mU/L, treatment with levothyroxine was started around the second week of life (18).
In neonates who had TSH concentrations between 6 and 20 mU/L, serum-based confirmatory tests were repeated beyond the age of 21 days, then if TSH was > 10 mU/L and T4 concentration was below 6.5 μg/dL, hypothyroidism was considered. In neonates with TSH > 10 mU/L and normal T4, confirmatory tests were further repeated 2 to 3 times with an interval of 2 to 4 weeks, and in cases with persistently elevated TSH (> 10 mU/L), replacement therapy with levothyroxine was considered (18).
In this study, all infants diagnosed with CH who were registered in the non-communicable diseases unit of the health department of Shiraz University of Medical Sciences were selected by the census method. Then the data on infants’ files, including gender, age, birth weight, height, type of delivery, parental consanguinity, maternal history of hypothyroidism, and TSH levels, were recorded in a researcher-made checklist.
3.4. Statistical Analysis
The data were analyzed using Statistical Package for the Social Sciences (SPSS) software, version 22.0 (IBM Inc., Chicago, IL, USA). Descriptive variables were presented as mean ± standard deviation (SD), frequency, and percentage. The normality of numerical variables was assessed using the Kolmogorov-Smironov and Shapiro-Wilks tests. The independent t-test, chi-square test, and Pearson correlation coefficient were used to evaluate the relationship between maternal hypothyroidism and the incidence of CH. Spearman’s rank correlation coefficient was used for variables with non-normal distribution. The odds ratio (OR) was calculated to evaluate the relationship between variables. A P-value less than 0.05 was considered statistically significant.
4. Results
Of 179,448 infants subjected to the national health care screening program for genetic, metabolic, and endocrine congenital diseases, including CH, from 2018 to 2020, 712 (4 in 1,000 live births) were diagnosed with CH (mean TSH: 22.34 ± 24.8 mlU/L). Overall, 35% of these infants had a history of maternal hypothyroidism.
Out of 712 neonates with CH, 370 (51.97%) were male; 323 (45.37%) were female, and 19 (2.66%) had unreported gender. The means of weight and height were 2752.54 ± 811.771 gr and 48.89 ± 4.529 cm, respectively.
Table 1 summarizes the demographic and clinical characteristics of hypothyroid infants and their relationship with maternal history of hypothyroidism. The age at the time of first sampling, weight, and head circumference at birth had normal distribution in hypothyroid infants born to healthy mothers.
| Maternal Hypothyroidism | No | Yes | P-Value |
|---|---|---|---|
| Infant gender | 0.732 b | ||
| Female | 209 (46.1) | 114 (47.5) | |
| Male | 244 (53.5) | 126 (52.5) | |
| Not recorded | 19 (2.66) | ||
| Type of birth | 0.019 b | ||
| Natural delivery | 183 (39.8) | 78 (31.0) | |
| Cesarean section | 277 (60.2) | 174 (69.0) | |
| Parental consanguinity | |||
| No | 265 (57.6) | 146 (58.0) | 0.920 b |
| Yes | 195 (42.4) | 106 (42.0) | |
| Infant’s age at the first sampling, mo | 4.79 ± 2.77 | 4.59 ± 2.43 | 0.341 c |
| Birth weight, g | 2733.9 ± 797.10 | 2777.72 ± 838.30 | 0.490 c |
| Birth height, cm | 47.80 ± 4.46 | 48.13 ± 4.61 | 0.370 c |
| TSH level, mlU/L | 24.23 ± 26.44 | 19.10 ± 21.30 | 0.027 d |
Neonates’ Characteristics (Born from 2018 to 2020) Based on the Mother’s History of Hypothyroidism a
The chi-square test showed that there was a strong and statistically significant relationship between the mother’s history of hypothyroidism and the occurrence of CH (OR = 4.951, P < 0.001).
Also, there was a significant relationship between the type of delivery and maternal history of hypothyroidism (OR = 1.47, P = 0.019).
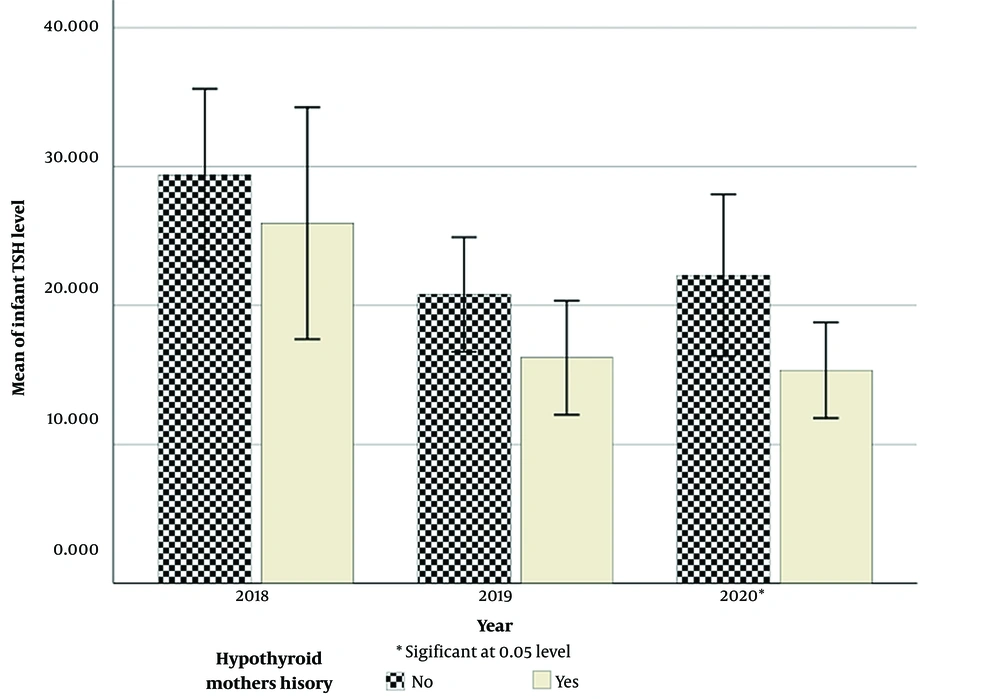
Moreover, the Mann-Whitney U test showed a statistically significant difference in the mean TSH level between hypothyroid infants born to mothers with a history of hypothyroidism and those who had healthy mothers (P = 0.027).
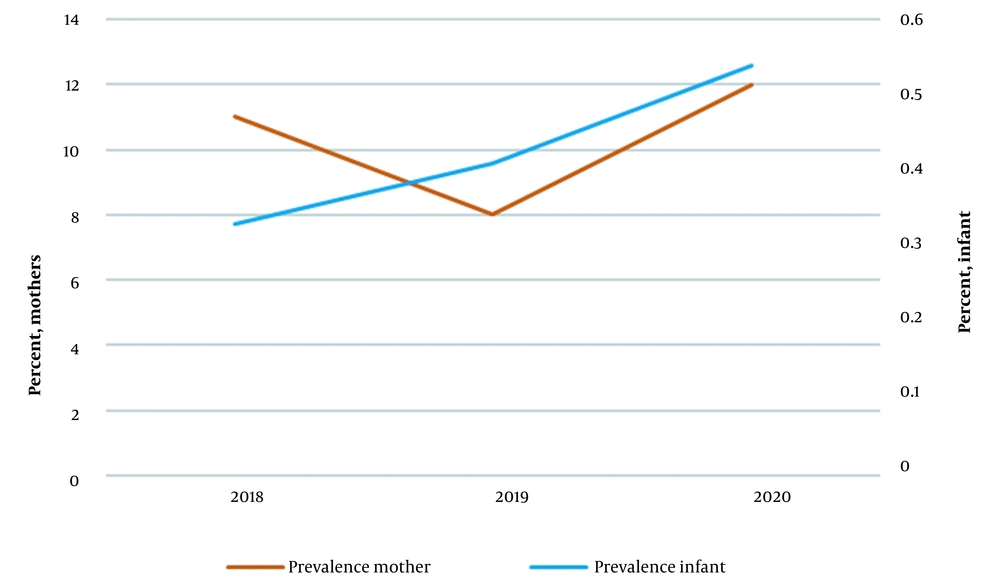
In 2018, 67447 infants (32553 girls and 34894 boys) were screened, 234 (0.34%) of whom had CH. Moreover, out of the total number of screened infants, 7371 (11%) had mothers with a history of hypothyroidism. In 2019, 58089 infants (27993 girls and 30096 boys) were screened, 233 (0.40%) of whom had hypothyroidism. Also, out of the total number of screened infants, 4905 (8%) had mothers with a history of hypothyroidism. In 2020, 53912 infants (25851 girls and 28061 boys) were screened. Of these, 245 infants (0.45%) had hypothyroidism, and 6509 (11%) had mothers with a history of hypothyroidism.
Figure 1 shows the trend of average levels of TSH in hypothyroid infants born to hypothyroid and healthy mothers between 2018 and 2020. Notably, infants whose mothers had hypothyroidism had lower TSH levels than infants whose mothers had no history of hypothyroidism. Moreover, Figure 2 shows the prevalence of neonatal and maternal hypothyroidism from 2018 to 2020.
5. Discussion
The present study showed a significant relationship between maternal hypothyroidism and CH. Also, in hypothyroid infants, those whose mothers had a history of hypothyroidism had lower TSH levels than infants whose mothers did not have a history of hypothyroidism. On the contrary, a study in Turkey showed that infants born to mothers with a history of thyroid disorders had higher levels of TSH (14). This difference is probably due to maternal treatment with levothyroxine, which explains the suppression of TSH in these infants. However, further investigations are recommended to explain such discrepancies and find other possible reasons. Contrary to the results of previous studies (16, 17) noting no significant relationship between maternal hypothyroidism and CH, the present study showed that the incidence of CH was 4 times higher in the infants of hypothyroid mothers compared to the neonates of healthy mothers. This difference may be due to different timing of performing thyroid functional tests (T4 and TSH), reflecting the residual effects of drug therapy during pregnancy on the normalization of neonatal tests’ results. Therefore, more studies are needed to elucidate the reasons for these controversial findings.
Our results showed no significant relationship between the gender of affected infants and the history of maternal hypothyroidism; however, the prevalence of CH was observed to be higher in boys than in girls. In a study in the United States, the incidence of CH increased in both genders similarly (19), which was similar to our observation. In contrast, most studies in other countries and Iran have reported female gender as a risk factor for CH, noticing a higher incidence of this condition in girls and women than in boys and men (20-22). Nevertheless, the reason for girls’ higher susceptibility to CH has not been understood yet. In a study in Finland, the incidence of CH was higher in girls than in boys (23). The reason for this difference may be related to a series of unknown environmental confounding factors that should be investigated in future studies.
Furthermore, the present study showed no significant relationship between the history of parental consanguinity and CH. Aligned with the results of our study, other studies in Iran and Brazil found no significant association between CH and parental consanguinity (24-26). This observation contradicts the findings of three studies in Iran, suggesting that parental consanguinity could be a probable cause of the high prevalence of CH (27-29). According to various reports, it is recommended to carry out studies to identify the environmental factors affecting the occurrence of CH, including consanguineous marriages.
In this study, the prevalence of CH among neonates born in Fars province was reported to be 0.34%, 0.4%, and 0.45% in 2018, 2019, and 2020, respectively, with an overall incidence of 4 per 1000 live births. This was similar to the results of a study in Yazd, Iran, noting that neonatal hypothyroidism was observed at a rate of 3.4% per 1000 live births (1 in 309 male infants and 1 in 286 female infants) (24). In another study in Fars province, this rate was reported to be 1:313.66 from 2013 to 2016 (30). The overall incidence of CH was reported as 1:2695 in Zurich (31) and 1:2000–1:4000 worldwide (4, 31). The prevalence of this disease in Iran is estimated to be higher than in other countries (4), which can be related to the relatively higher prevalence of iodine deficiency, especially moderate iodine deficiency, among Iranian pregnant women since 1968 (32). Due to the high prevalence and increasing trend of this disease in our country, it is necessary to pay more attention to neonatal screening programs in the first month of birth and follow-up tests for at-risk infants.
5.1. Limitations
Since this study was conducted in Fars province, the generalization of the results to other groups in society should be made with caution. Moreover, because of incomplete data registration by healthcare providers, we had to exclude the data of some patients, highlighting the need for appropriate training of healthcare staff with regard to the importance of accurate data registration into the health information system.
5.2. Conclusions
Our results showed a significant relationship between maternal hypothyroidism and CH, but no such relationships were found for gender, parental consanguinity, age, and birth weight. Therefore, considering the importance of prompt diagnosis of CH, especially in high-risk infants, such as those born to hypothyroid mothers, it is recommended to conduct new studies to develop more precise neonatal screening guidelines so that by the early diagnosis and timely treatment of the disease, we can reduce its complications.