1. Background
Rationale: Premature ovarian insufficiency (POI) is defined as a decline in ovarian function before age 40 (1). It is among the major etiologies of female infertility. According to the guideline presented by the European Society of Human Reproduction and Embryology (ESHRE), POI can be diagnosed based on the following criteria: Patient being under 40 years of age, history of at least 4 months of oligo/amenorrhea, and elevated serum Follicle-stimulating Hormone (FSH) (higher than 25 mIU/mL) in two measurements with at least 4 weeks of interval (2).
The etiologies of POI are still unknown, but they may include genetic, iatrogenic, and autoimmune factors, unhealthy lifestyle, and psychological stress (3, 4). Genetic factors do not fully determine the age of menopause. Recent studies have reported that modifiable lifestyle factors, including diet, may affect ovarian aging (5-7). Currently, the main treatment for POI is hormone replacement therapy (HRT). As known, HRT can improve low estrogen-induced clinical complications but does not affect ovarian function or fertility (8, 9). Furthermore, long-term HRT might increase the risk of hormone-related disorders, including breast and endometrium disorders and thrombotic events (10). Therefore, more efficient treatment strategies are needed for POI.
Fat-soluble vitamins, including vitamins A, D, and E, have various physiological functions (11). These vitamins have different effects, including antioxidant function, enzyme cofactor, and precursor for steroid hormones (12). Some studies have reported that fat-soluble vitamin deficiencies may cause POI (13-15). Recent studies have correlated low serum vitamin D3 levels with degrees of infertility, metabolic diseases, and endocrine disorders, including polycystic ovary syndrome, premature ovarian failure (POF), and ovarian cancer (15-17). Animal and human studies have shown that vitamin D3 receptors and its metabolic enzymes are present in some genitourinary tissues, including the vagina, ovary, uterus, fallopian tube, and placenta (15, 18, 19). Therefore, it is hypothesized that VD3 may directly affect these organs (15, 18, 19). Vitamin D converts to its active form, 1,25-dihydroxyvitamin D3 (20, 21). One of the target organs of vitamin D is the reproductive organs, including the ovaries, that express vitamin D receptors.
Furthermore, vitamin D is a precursor for many lipid-soluble steroidal hormones and, thus, may regulate the female reproductive system. Regardless of its effects on ovarian function, vitamin A has not yet been associated with ovarian insufficiency. However, an inverse association was reported between the serum vitamin A/TC ratio and the risk of POI (13). Therefore, serum vitamin A/TC ratio may be used to predict POI. Based on this assumption, vitamin A supplementation might be beneficial in preventing or treating POI (13). It was recently reported that administration of selenium and vitamin E supplements increased AMH levels, the number of antral follicles, and ovarian volume in women with occult POI (22).
Some studies have pointed out that besides its adverse effects on health, POI is associated with an increased risk of premature mortality, deficits in cognition, osteoporosis, and cardiovascular diseases in women (23-25). Moreover, POI may have a substantial economic and psychological burden regarding family planning, especially in cases where women choose to delay their childbearing to later reproductive years (26). As vitamin deficiencies are preventable and curable, documenting the relationship between serum levels of fat-soluble vitamins and POI can establish these vitamins as preventable factors contributing to POI. However, previous studies have found controversial results regarding the association between fat-soluble vitamins in general and each fat-soluble vitamin and POI (13, 17, 27).
2. Objectives
This systematic review evaluated the relationship between serum levels of fat-soluble vitamins and POI.
3. Methods
3.1. Identification and Selection of Articles
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist was used to prepare this systematic review (28). The review was performed on studies evaluating the relationship between plasma levels of fat-soluble vitamins and POI. The plasma level of fat-soluble vitamins is used as an indicator of their status. This is because the serum level of fat-soluble vitamins reflects the adequacy of dietary intake for vitamins A, K, and E, as well as the adequacy of dietary intake and skin synthesis for vitamin D (29).
3.2. Search Strategy
The article search was performed online in PubMed, Web of Science, and Scopus until June 2022. Google Scholar was also searched as a grey literature source. There was no time or language restriction for retrieving the articles. The search terms included fat-soluble vitamins, premature ovarian insufficiency, and premature ovarian failure. The search strategy was based on medical subject headings (MeSH) terms. Furthermore, a manual search was performed using the keywords in combination with Boolean operations (AND and OR) (Table 1, Appendix 1 and 2). Furthermore, a manual search of the references of the identified articles was performed to identify possible related articles.
| PICO Compartment | Search Term | Keywords |
|---|---|---|
| Intervention/Exposure | Fat-soluble vitamins | Fat-soluble vitamin; fat-soluble vitamins; vitamin A; retinol; vitamin D; vitamin E; tocopherol; alpha-tocopherol; vitamin K; menadione |
| Comparison | ||
| Outcome | Premature ovarian failure | Premature ovarian failure; premature ovarian insufficiency; premature menopause |
3.3. Inclusion and Exclusion Criteria
All electronically published observational studies, namely cohort, case-control, case series, and case reports in peer-reviewed journals until the end of June 2022, and studies on the plasma levels of fat-soluble vitamins and POI were included in the review. The search was performed in June 2023. Articles in English and Persian were included. The review excluded comments, letters to editors, and studies with low evidence levels. Two independent researchers performed the search. A third researcher was invited to solve the discordance in case of disagreement.
3.4. Study Selection
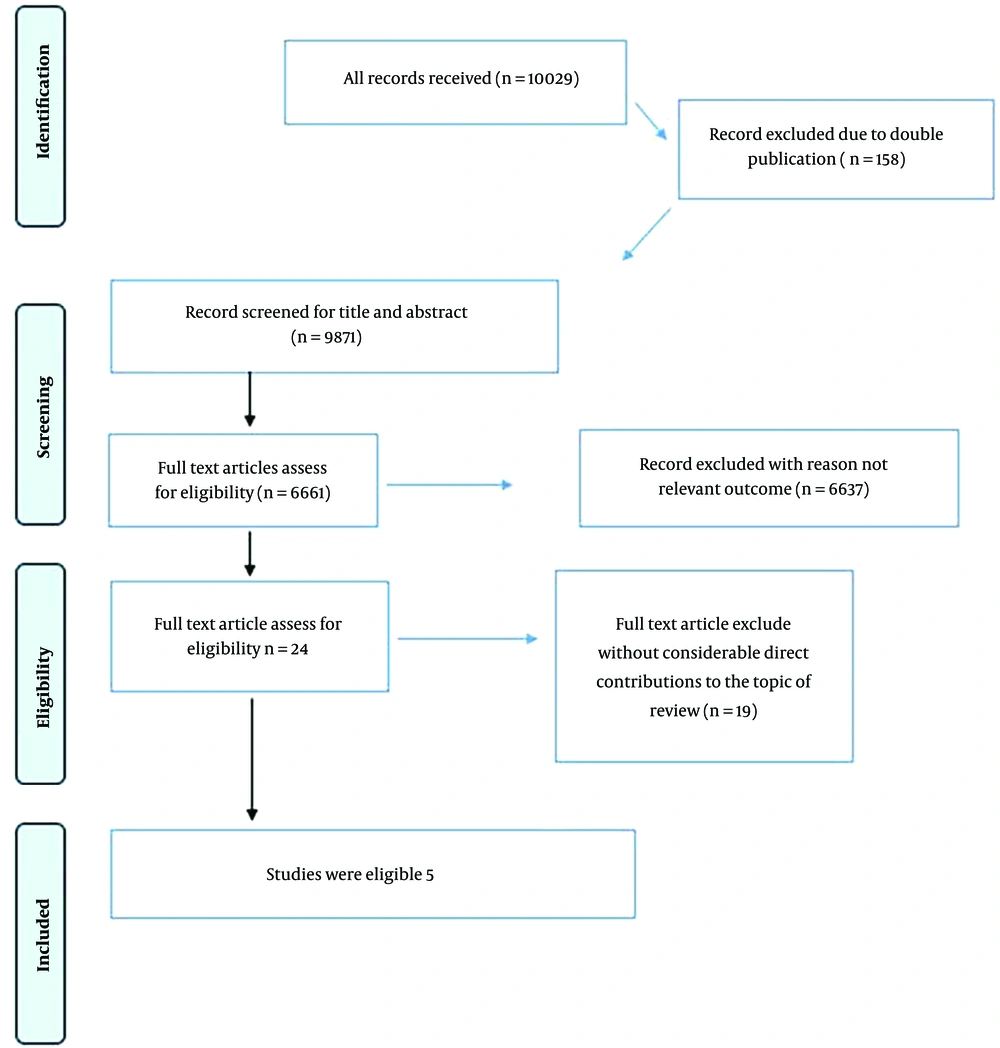
A total of 10,029 articles (3,443 from Scopus, 76 from PubMed, 632 from Web of Science, and 5,868 from Google Scholar) were retrieved. Furthermore, 10 articles were identified by reviewing the retrieved studies' references. Among the retrieved studies, 158 duplicate articles were excluded, resulting in the remaining 9,871 articles. After applying the inclusion criteria on titles and abstracts, 6,661 articles were excluded. Then, the full-text eligibility of the remaining 3,210 articles was assessed. After evaluating full-text articles for eligibility, 19 were excluded based on the exclusion criteria and low quality. Finally, 5 studies were found eligible. The article selection procedure is summarized in the flow chart presented in Figure 1.
The identified articles were selected independently by two reviewers (SD and RGH), and the studies' data were extracted in researcher-made forms. Finally, any disagreement between the reviewers was resolved by consensus between the reviewers.
The included observational studies evaluated 25(OH) D, vitamin E, and vitamin A serum level assays and analyzed the association between the serum levels of these vitamins and POI.
3.5. Quality Assessment
The quality assessment was performed based on the Newcastle-Ottawa Quality Assessment Scale without considering the objectives of the studies (30). Each item is granted one score, and the sum of scores is used for quality assessment (31). This scale has different items for study types. The scale for case-control studies includes items on selection (4 items), comparability (one item), and exposure (3 items); the items in cohort study evaluation include selection (4 items), comparability (one item), and outcome (4 items), and the scale for cross-sectional study evaluation scale includes items on selection (4 items), comparability (one item), and outcome (two items). Considering the types of studies included in this review, three versions of the scale were used. As the scales yield different scores, in this review, each study's score was converted into a percentage to have a similar percentage score for different studies. The total score of the scale was used to characterize the studies into high-quality (studies that scored 75% of the total score), moderate quality (studies that scored between 50% and 75% of the total score), and Low-quality (studies that scored less than 50% of the total score). Therefore, low-quality articles were excluded based on the 50% cut-off in this review. Two researchers (SD and ZF) evaluated the quality of the articles independently. Disagreements between the reviewers were resolved through discussion between the reviewers (AT). If no agreement was achieved between the reviewers, the conflict was resolved by consulting the first author.
4. Results
The characteristics and findings of the reviewed articles are summarized in Table 2. The included studies had NOS scores higher than 75% of the total score. Among the five studies, three studies evaluated serum vitamin D (15, 17, 27), one study included serum vitamin A (13), and one study included serum vitamin E (14).
| Author | Year | Country | Design and Sample Size | Age Range | N | Method Used for Assessing Serum | Conclusion |
|---|---|---|---|---|---|---|---|
| Purdue-Smithe et al. (17) | 2018 | US | Nested case (328) control (328) | 25 - 42 | 656 | Plasma blood samples: Total 25(OH)D; free 25(OH)D; vitamin D-binding protein | No significant relationship between the risk of premature menopause and serum level of Total (OR = 1.04, CL = 95%, CI (0.60, 1.80) and free vitamin D ( OR = 0.70, CL = 95%, CI[0.41, 1.20]); Indirect relationship between serum level of VDBP and premature menopause (OR = 1.80, CL = 95%, CI [1.09, 2.98]); |
| Ersoy et al. (27) | 2014 | Turkey | Cross-sectional | 22-39 | 130 | ELISA: 25(OH)D3 | The serum level of vitamin D (based on the ELISA method) was 7.75 [3-21.22] μg/L in the POF group and 6.74 [3-25.54] μg/L in the control group (P = 0.477). No relationship was found between serum level of vitamin D and POF etiology. |
| Chen et al. (13) | 2022 | China | Case (47) – control (67), cross-sectional study | 18 - 40; >40 (8 women) | 114 | Reverse-phase high-performance liquid chromatograph: Serum vitamin A; total cholesterol | Plasma vitamin A was higher in the POI group (728.00 ± 176.00 µg/L) than in the control group (503.93 ± 145.64 µg/L) (P = 0.13). ; An inverse relationship between vitamin A/TC ratio and the risk of POI (143.14 ± 35.86 vs. 157.56 ± 35.21 µg/mmol, P = 0.04, OR = 0.988, 95% CI for OR: 0.977 – 0.999, P = 0.04) |
| Ma et al. (14) | 2021 | China | Case (40); control (56) | <40 | 96 | ECLIA: Serum vitamin E; total cholesterol | Lower serum level of vitamin E in the POI group (1.52 ± 0.27 mg/mmol) than in the control group (1.68 ± 0.36 mg/mmol) (P = 0.011) |
| Kebapcilar et al. (15) | 2013 | Turkey | Case (35); control (28); cross-sectional | < 40 | 63 | High-performance liquid chromatography: 25(OH)D | Serum vitamin D in the POF and control groups was 9.5 ± 4.0 and 18.5 ± 7.5 ng/mL, respectively (P < 0.001); significant inverse relationship between serum vitamin D level and the risk of POF |
4.1. Relationship Between Serum Vitamin D and POI
Purdue-Smithe et al. prospectively evaluated the relationship between serum level of total and free 25(OH)D and its binding protein (VDBP) and POI risk. This nested case-control study included 656 women aged 25 to 42. The serum 25(OH)D quartiles were determined in the case and control groups. The risk of POI was not significantly related to serum level of total (OR = 1.04, 95% CI for OR [0.60, 1.80]) and Free 25(OH)D (OR = 0.70, 95% CI for OR [0.41, 1.20]), but was indirectly related to serum level of VDBP (OR = 1.80, 95% CI for OR [1.09, 2.98]) (17).
Ersoy et al. evaluated the prognosis of vitamin D for POI in a cross-sectional study of 48 women with POI and 82 women as the control group. The ELISA method was used to measure serum levels of 25(OH)D. No significant relationship existed between serum vitamin D level and POI (P = 0.477) (27).
Kebapcilar et al. evaluated serum vitamin D in POI women in a case-control study of 63 women, including 35 women with POI, in 2013 in Turkey. They reported a significant and inverse relationship between serum vitamin D level and the risk of POI (9.5 ± 4.05 ng/mL in the POI group and 18.5 ± 7.5 ng/mL in the control group; P < 0.001) (32).
4.2. Relationship Between Serum Vitamins A and E and POI
In a cross-sectional study, Chen et al. evaluated the relationship between serum levels of vitamin A and POI in 2022. They collected samples from 130 women from 2016 to 2018 in China, among whom 47 had POI. They measured serum levels of vitamin A, estradiol (E2), luteinizing hormone (LH), FSL, and anti-Mullerian hormone (AMH). They found slightly higher serum vitamin A levels among the POI group than the control group, but the difference was not statistically significant. They also calculated the vitamin A/TC ratio and identified a significant and inverse relationship between this ratio and the risk of POI (OR = 0.988, 95% CI for OR: 0.977 – 0.999, P = 0.04). They hypothesized that the vitamin A/TC ratio could predict POI and that vitamin A supplementation may be used to prevent or treat POI (13).
In a case-control study in 2021, Ma et al. evaluated the role of vitamin E in the etiology of POI in Chinese women. They collected blood samples from 96 women, including 40 women with POI, and measured serum levels of vitamin E. They reported that the serum level of vitamin E was significantly lower in the POI group than in the control group (1.52 ± 0.27 mg/mmol for POI and 1.68 ± 0.36 mg/mmol, P = 0.011) (14).
5. Discussion
This systematic review investigated 5 case-control and cross-sectional studies on the relationship between serum levels of fat-soluble vitamins and POI. In three studies, the authors evaluated the relationship between free and total serum levels of vitamin D and POI. Although no direct relationship was observed between serum vitamin D levels and POI, one study reported an indirect relationship between VDBP and POI serum levels. Furthermore, another study reported a significant relationship between serum levels of vitamin D and POI. In terms of serum vitamin A and E and POI, a significant relationship was reported between serum vitamin E and vitamin A/TC ratio and POI, while no direct relationship was reported between vitamin A and POI.
One feature of POI is elevated serum levels of gonadotropins (33). The AMH is a hormone that prevents the deployment of primary follicles and preserves ovarian reserves (34). This hormone regulates follicular growth by inhibiting their sensitivity to FSH. Thus, low AMH serum level is an important indicator for POI (33). Studies have shown that vitamin D3 may increase the synthesis of AMH and thus adjust follicular growth through regulating intracellular signal pathways (34). On the other hand, vitamin D deficiency may decrease the serum level of AMH and increase FSH concentration, leading to POI (27). In a prospective study on 116,430 nurses (25 - 42 years) in Boston, USA, no significant relationship was reported between total and free 25(OH) D3 and early menopause and AMH level; in contrast, high serum level of VDBP was associated with low serum level of AMH and increased risk of early menopause (17). Another study on 48 women with POI and 82 controls in Turkey in 2014 showed no significant difference in serum vitamin D levels between the groups. The study did not investigate the relationship between serum vitamin D levels and FSH and E2 (27). On the other hand, in a cross-sectional, control-case study performed on 35 women with POI and 28 women with normal menstruation cycles in Turkey in 2013, serum vitamin D level was lower in the POI group than in the normal group. The study reported a reverse relationship between serum vitamin D level and FSH serum level.
Conversion of primordial to primary follicle produces a large amount of reactive oxygen species (ROS) that may result in an imbalanced oxidant-antioxidant situation and oxidative stress (22, 35). Oxidative stress can cause apoptosis in granulosa cells and result in infertility and POI (22, 36). Selenoprotein enzyme GPX1 is the most important antioxidant enzyme that scavenges ROS in the ovary. Selenium and vitamin E are the cofactors for GPX1 (37). In a case-control study of 40 women with POI and 56 women as the control group, a negative correlation was reported between serum α-Tocopherol level and FSH and LH and a positive correlation between α-Tocopherol level and AMH in both the case and control groups. They also found that serum α-Tocopherol level was lower in POI women than in the control group (14).
Vitamin A can affect follicular growth, ovarian steroidogenesis, oocyte maturation, and corpus luteum formation (38-40). Therefore, the serum level of vitamin A can be related to the quality of human oocytes in follicular fluid (41). A cross-sectional study of 47 women with POI and 67 women as the control group showed the POI groups had a slight and non-significantly higher serum vitamin A level than the control group, which was not statistically significant. In addition, the vitamin A/TC ratio was inversely related to the risk of POI (13).
The strength of this study was the inclusion of studies that evaluated the relationship between POI and fat-soluble vitamins. The study combined the overall findings regarding this group of vitamins concerning POI. This review had some limitations. First, the evaluated articles differed regarding the commercial kits they used for measuring serum vitamin levels (42). Second, many factors, including seasonal differences, sunscreen creams, geographic region, race, skin color, lifestyle, and nutrition, could influence the amount, synthesis, and availabilities of vitamins, and the reviewed studies did not consider all these influencing factors when assessing the relationship between serum level of fat-soluble proteins and POI (43, 44). Another limitation of this review was the language barrier that prevented the inclusion of articles published in languages other than English and Persian.
Although it has been reported that vitamins D, E, and A play a role in ovarian physiology, the findings of this review could not justify or reject the hypotheses regarding the role of vitamins in the etiology of POI. Therefore, extensive studies should be conducted worldwide to clarify the exact mechanism of POI and the role of vitamins in its etiology and treatment. If the predictive value of fat-soluble vitamins for POI is documented, more aggressive treatment for deficiency in these vitamins might be considered in preventing POI in high-risk individuals.
In conclusion, fat-soluble vitamins take part in various physiological functions. These vitamins have various effects, including antioxidant functions, acting as cofactors, and serving as precursors for steroid hormones. Therefore, they do not have any detrimental effects on receptor organs. The ovary is one of these organs, and a deficiency in fat-soluble vitamins causes premature ovarian insufficiency. Based on the findings of this study, at least some fat-soluble vitamins or their protein binding might be related to POF. However, it has been reported that vitamins D, E, and A play a role in ovarian physiology, there was not enough scientific evidence to prove the relationship between serum fat-soluble vitamins and POF. Extensive studies, including meta-analyses, should be conducted worldwide to clarify the exact mechanism of POF and the effect of vitamins in etiology and treatment. This study proved the possible relationship between serum levels of some fat-soluble vitamins and POF. If documented in further studies, correction of fat-soluble vitamin deficiencies might serve as preventive or curative strategies for POF.