1. Background
Labor involves several changes in cervical function, and before the onset of labor contractions, the cervix must undergo a more extensive transformation. Cervical remodeling primarily includes soft tissue changes, known as cervical ripening, which is one of the main phases in the onset of labor (1, 2). Shortening the duration of labor is a valuable achievement in midwifery, especially in cases where continuing the pregnancy poses serious risks to the mother and fetus due to reasons such as severe pre-eclampsia, cardiovascular diseases, or prolonged pregnancy (1).
As one of the most common obstetric interventions used to reduce the duration of labor, induction is performed when its benefits to the mother and fetus outweigh the risks of continuing the pregnancy (3). In 1964, Bishop introduced a standardized scoring system to assess cervical ripening. This cervical assessment system is based on scoring five criteria: Cervical dilatation, effacement, station of the presenting fetal part, position, and the consistency of the cervix during the vaginal examination. Each of the first three criteria is scored from 0-3, and each of the next two criteria is assigned a score of 0 - 2. According to this scoring system, the maximum Bishop score is 13 (4, 5).
Therefore, before selecting the appropriate treatment regimen for labor induction, cervical ripening should be assessed by calculating the Bishop score. A higher Bishop score indicates a riper and more favorable cervix, increasing the probability of vaginal delivery and reducing the rate of cesarean section and its associated complications (4-8). Previous studies have shown that a low Bishop score (≤ 6) is associated with prolonged labor pain and an increased risk of cesarean delivery (7-9).
The results of one study in 2015 demonstrated that the Bishop score increased significantly when a cervical softener was used, leading to a reduction in delivery time (4). Various pharmacological and mechanical methods can facilitate cervical ripening (10-14).
Medicinal herbs are one of the alternative and complementary methods used to enhance the labor and childbirth process. Despite significant advances in medical science, the use of herbal medicines, especially in pregnancy care, has a long history worldwide. The consumption of medicinal herbs in developing countries, such as Iran, is higher than in other countries, possibly due to their accessibility, abundance, and cultural diversity (15-17). Women are among the main consumers of complementary medicine and medicinal herbs, often choosing these methods during pregnancy due to concerns about the toxicity of synthetic drugs and the belief that medicinal herbs are safer for the fetus than chemical drugs, which often lack evidence-based support (18, 19).
The use of medicinal plant compounds during pregnancy requires a sound understanding and adherence to consumption standards based on research involving pregnant women (20, 21). Consequently, the World Health Organization has encouraged its member states to conduct research and raise awareness about medicinal herbs and traditional medicine (22).
Evening primrose possesses phytoestrogenic properties and contains the precursor to prostaglandin (13). Previous studies have demonstrated that evening primrose oil is rich in linoleic acid, a key component responsible for its prostaglandin content. The use of primrose oil significantly elevates gamma-linolenic acid (GLA) levels in the blood, thereby enhancing the biosynthesis of prostaglandin E1, prostaglandin E2, and arachidonic acid. Notably, prostaglandin E1 is a recommended medication for cervical ripening (23, 24).
It is worth noting that low Bishop scores and an unripe cervix have been associated with an increased risk of adverse childbirth outcomes, according to previous studies (25-27). Given the frequent use of labor induction in midwifery, one crucial and effective factor for the success of labor induction is the improvement of the Bishop score. The enhancement of the Bishop score can potentially reduce the rate of cesarean deliveries, aligning with the policies of both the World Health Organization and the Ministry of Health in Iran (26).
Additionally, numerous studies have demonstrated the effectiveness and safety of evening primrose oil in positively influencing the Bishop score for both mothers and infants (25). Nevertheless, no previous study has directly compared the effects of oral and vaginal evening primrose capsules administered simultaneously with labor induction. The examination of the impact of a single dose of vaginal or oral evening primrose capsules administered concurrently with labor induction, as opposed to a prolonged administration at the end of pregnancy, on the Bishop score, labor duration, cesarean section rates, and maternal and neonatal outcomes holds paramount significance in obstetrics. Therefore, this study was conducted due to the importance of promoting natural childbirth and its impact on the childbirth process, in addition to the absence of prior research in this specific area.
2. Objectives
The present study aimed to compare the effects of oral and vaginal evening primrose capsules with concurrent labor induction and exclusive labor induction on bishop score and some labor outcomes.
3. Methods
This study was a triple-blind randomized clinical trial and was registered with the ethics code IR.RUMS.REC.1398.122 and clinical trial code IRCT20190717044248N4.
3.1. Study Population
The study population for this study comprised pregnant mothers who were admitted to Niknafs Maternity hospital in Rafsanjan, Iran, for labor induction in 2020. The participants were selected based on specific inclusion and exclusion criteria.
3.2. Inclusion and Exclusion Criteria
The inclusion criteria were written informed consent to participate in the study, a gestational week at or over 38 weeks, maternal age between 18 and 35 years, a healthy amniotic sac, no labor contractions, a low-risk pregnancy (which excludes conditions such as placental abruption, diabetes, or pre-eclampsia), a singleton fetus in a cephalic presentation as determined by the last ultrasound, an estimated fetal weight within the range of 2500 - 4000 g, Bishop score equal to and less than 6 (7), Iranian nationality, and no diagnosis of chronic diseases. Participants were excluded from the study if they required emergency interventions due to maternal or fetal reasons after the start of induction (e.g., umbilical cord prolapse or placental abruption), engaged in unprotected intercourse, or used other herbal remedies.
3.3. Sample Size and Sampling
The sample size was determined based on the formula used in the study by Kalati et al. as follows (26):
In this formula, σ is the standard deviation (SD) of the Bishop score in the 2 study groups, which is equal to 2.8 in a similar study (26), and d is considered the minimum significant difference in terms of the study objectives, which is equal to 1.8. Finally, with a confidence level of 0.95 and a test power of 0.90, the final sample size was calculated as 57 subjects in each group (calculated by adding dropout, with 10% dropout).
Random allocation was performed using the minimization method. Among the individuals admitted to the maternity hospital for labor induction, those meeting the inclusion criteria were selected as the research sample. To assign individuals to either group A or group B, the initial participant in each stratum was randomly chosen by the flip of a coin. Subsequent samples in each stratum were allocated to the group with the lower sum of the number of pregnancies and Bishop score within that stratum. In case of equal sums, a coin toss was repeated. The randomization sequence was determined by a statistics specialist. An experienced midwife assisted in enrolling participants; however, a non-midwife, not involved in labor procedures, assigned participants to interventions. The participants were divided into three groups: Oral evening primrose oil (n = 57), control (n = 57), and vaginal evening primrose oil (n = 57).
After obtaining the necessary approvals, the researcher visited Niknafs Maternity hospital in Rafsanjan to identify pregnant women admitted to the hospital. These women were diagnosed by an obstetrician for labor induction due to various reasons, such as abnormal electrocardiogram (ECG), reduced fetal movement, decreased amniotic fluid, post-term pregnancy, or a request for termination of pregnancy. They also had to meet the inclusion criteria.
Before commencing the intervention, pregnant mothers who met the inclusion criteria underwent a vaginal examination by the research associate. If they were eligible, they were referred to another project associate, who was not a midwife and was not currently in labor, to receive medication. The need for cesarean delivery was determined by an obstetrician in this study. Additionally, the participants, experimenters (responsible for drug distribution and vaginal examinations), and the researcher analyzing the data were all blinded to the treatment assignments.
3.4. Ethical Considerations
The research objectives and procedures were thoroughly explained to the subjects, and written informed consent was obtained from all participants. The participants were assured that their involvement in the study would not prevent them from receiving conventional treatment at the maternity hospital. They were informed that they had the freedom to withdraw from the study at any stage, and their information would be kept confidential.
3.5. Interventions
The intervention was carried out as follows:
The first intervention group received a package containing two 1 000 mg oral evening primrose capsules (both capsules were bought from Barij Pharmaceutical Company, Iran, and taken simultaneously at the onset of induction) (26), and the second intervention group received two 1 000 mg vaginal evening primrose capsules (both simultaneously at the onset of induction) (25), along with the induction, which was safe for mothers and neonates according to previous studies (25, 27-30). The control group did not receive any special medication related to evening primrose and underwent induction alone. In all cases, induction was performed using 10 units of oxytocin per 1000 cc of Ringer’s serum, following the obstetrician’s instructions, and the induction procedure was identical across all 3 groups (31).
Subsequently, vaginal examinations were conducted again by the project associate 2 and 4 hours after induction, and the findings were recorded in the respective files. Information about the time elapsed from the initiation of labor induction to the onset of contractions, the commencement of the active phase, Bishop score, duration of the first and second stages of labor, mode of delivery, presence of meconium, and the newborn’s Apgar score were retrieved from the hospital records on the morning following delivery by another researcher and documented in the relevant checklist.
3.6. Data Collection Tools
The research tools included a checklist whose content had been approved by 10 university professors. The checklist consisted of three parts: The first part covered the mother’s demographic characteristics (e.g., age, education level, occupation, and place of residence), the second part addressed obstetric characteristics (e.g., height, weight, gestational age, current pregnancy type, history of abortion, Bishop score at admission, estimated fetal weight, date of capsule initiation, potential side effects resulting from capsule use, and Bishop score 1 week before capsule ingestion), and the third part focused on childbirth characteristics (e.g., date of childbirth, first-stage labor characteristics, second-stage labor characteristics, third-stage labor characteristics, neonate’s weight, neonate’s gender, and amount of bleeding). The validity of the checklist was confirmed through the content validity method.
To maintain blinding, Barij Pharmaceutical Company supplied the drugs in packages labeled as group A and group B. Drug distribution was conducted by an uninformed individual who was not involved in the delivery process. Consequently, both the participants and research colleagues responsible for vaginal examinations, drug distribution, and data recording and the statistical experts remained unaware of the types of drugs administered to the study groups. The primary outcome was the mean Bishop score; however, the secondary outcome was the duration of the first stage of labor.
3.7. Data Analysis
The data were imported into SPSS software (version 24; IBM, Chicago, IL, USA). Descriptive statistics, including frequency, percentage, mean, median, range, and SD, were reported. The Kolmogorov-Smirnov test was employed to assess the normality of quantitative variables. One-way analysis of variance in repeated measurements (with Tukey post-hoc test) and independent t-tests were used for comparing means between the two groups (the Kruskal-Wallis test and the Mann-Whitney test for non-parametric variables). Additionally, the chi-square test was utilized to compare the proportions of qualitative variables between the 2 groups. A significance level of P < 0.05 was considered.
4. Results
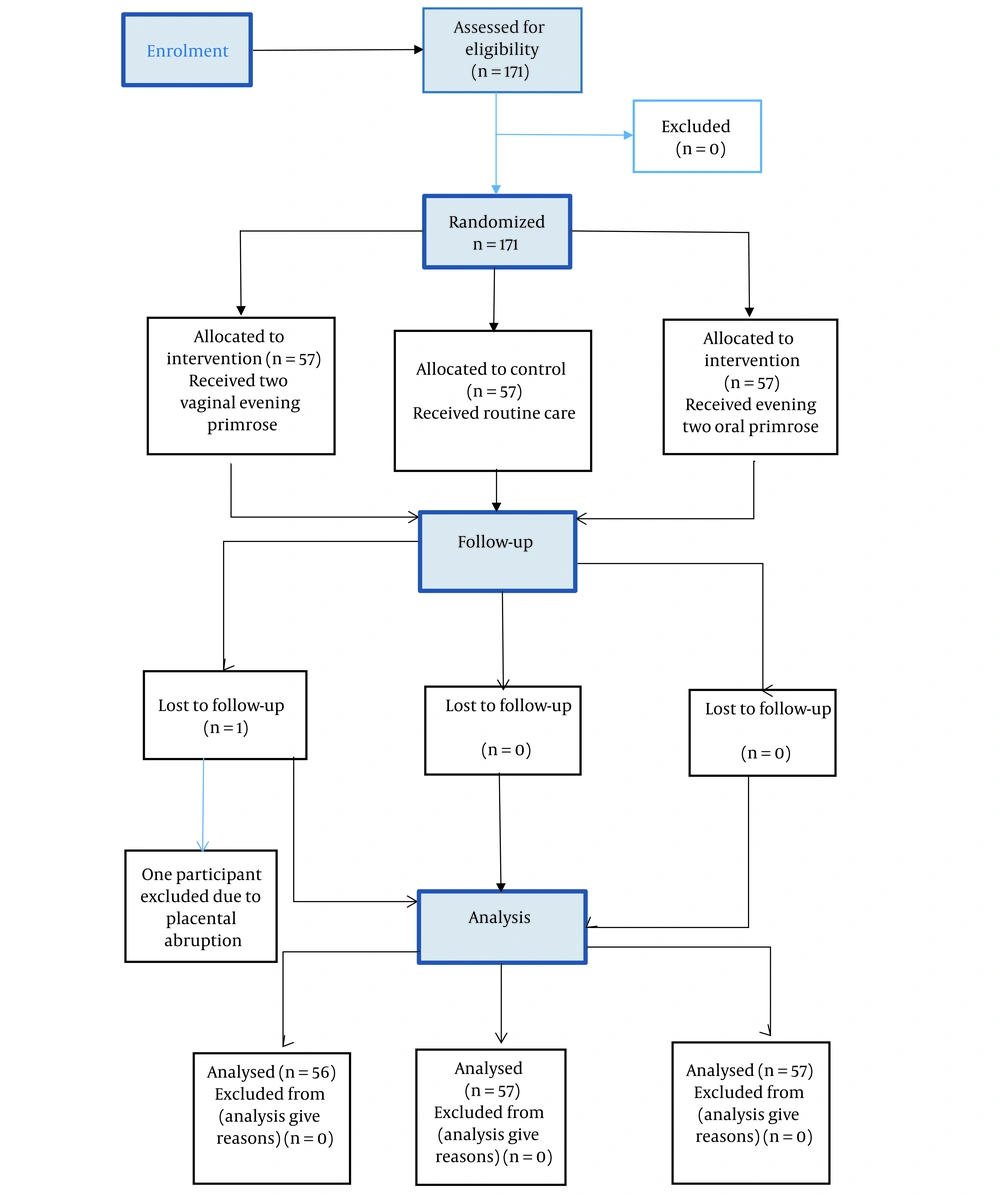
In this study, 171 pregnant mothers were randomly assigned to three groups: Oral evening primrose oil (n = 57), control (n = 57), and vaginal evening primrose oil (n = 56, with 1 participant excluded due to placental abruption) (Figure 1). There were no significant differences among the three groups in terms of demographic characteristics such as age, body mass index (BMI), gestational age, and gravidity. Gestational age, BMI, and age followed a normal distribution according to the Kolmogorov-Smirnov test (Table 1).
| Variables and Groups | Oral Evening Primrose (N = 57) | Vaginal Evening Primrose (N = 56) | Control Group (N = 57) | The Value of the Statistical Index of the Test | P-Value a |
|---|---|---|---|---|---|
| Quantitative Variables; Mean ± SD (Range) | |||||
| Age (y) | 27.28 ± 4.68 (18 - 35) | 28.73 ± 4.72 (20 - 35) | 27.56 ± 4.59 (18 - 34) | F = 1.535 | 0.218 b |
| BMI (kg/m2) | 30.36 ± 4.29 (20.83 - 41.23) | 28.87 ± 4.17 (19.78 - 42.22) | 28.99 ± 4.56 (14.19 - 39.56) | F = 2.069 | 0.130 b |
| Gestational age based on the LMP (week) | 39.53 ± 1.18 (36.3 - 42.0) | 39.39 ± 0.92 (37.0 - 41.0) | 39.29 ± 1.10 (36.4 - 41.6) | F = 0.678 | 0.509 b |
| Gestational age based on first-trimester ultrasound (week) | 39.53 ± 1.00 (36.50 - 42.00) | 39.28 ± 1.30 (37.30 - 41.00) | 39.17 ± 1.13 (37.00 - 41.00) | F = 1.994 | 0.139 b |
| Qualitative Variables; No. (%) | |||||
| Gravidity | χ2 = 0.081 | 0.960 c | |||
| Primigravida | 30 (52.6) | 28 (50.0) | 29 (50.9) | ||
| Multigravida | 27 (47.4) | 28 (50.0) | 28 (49.1) | ||
Abbreviations: SD, standard deviation; BMI, body mass index; LMP, last menstrual period.
a P < 0.05, the difference is statistically significant.
b One-way analysis of variance.
c Chi-square test.
The median time interval from the onset of induction to the onset of labor contractions, the time interval from the onset of induction to the onset of the active phase, the duration of the active phase of labor, the duration of the first phase, the duration of the second phase, and the time from the onset of induction to delivery in the three groups under study had a statistically significant difference (P < 0.001) (Table 2).
| Variables and Groups | Oral Evening Primrose (n = 57) | Vaginal Evening Primrose (N = 56) | Control Group (N = 57) | The Value of the Statistical Index of the Test | P-Value |
|---|---|---|---|---|---|
| The time interval from the onset of induction to the onset of labor contractions (min); median (range) | 80 (30 - 120) | 30 (28.75 - 60) | 180 (120 - 290) | H = 72.338 | < 0.001 a |
| The time interval from the onset of induction to the onset of the active phase (min); median (range) | 120 (60 - 212.50) | 60 (30 - 97.50) | 290 (200 - 400) | H = 68.411 | < 0.001 a |
| The time from the onset of induction to delivery (min); median (range) | 300 (188.75 - 381.25) | 210 (135 - 280) | 487.50(381.25 - 787.5) | H = 61.461 | < 0.001 a |
| Duration of the first phase of labor (min); median (range) | 255 (176.25 - 360) | 190 (120 - 250) | 450 (735 - 360) | H = 60.206 | < 0.001 a |
| Duration of the second phase of labor (min); Median (range) | 15 (10 - 41.25) | 10 (7.50 - 30) | 30 (15 - 47.50) | H = 15.345 | < 0.001 a |
| Apgar score at first minute (variation range); mean ± SD (range) | 8.70 ± 1.27 (0 - 9) | 9.47 ± 1.01 (9 - 10) | 8.84 ± 1.53 (6 - 9) | F = 1.990 | 0.140 b |
| Apgar score at fifth minutes (variation range); mean ± SD (range) | 9.89 ± 0.41 (8 - 10) c | 9.95 ± 0.19 (9 - 10) | 9.93 ± 0.37 (8 - 10) | F = 1.613 | 0.202 b |
| Type of delivery; No. (%) | F = 4.253 | 0.373 d | |||
| Normal | 44 (77.2) | 49 (87.5) | 47 (82.5) | ||
| Vacuum | 2 (3.5) | 0 | 3 (5.3) | ||
| Cesarean section | 11 (19.3) | 7 (12.5) | 7 (12.3) | ||
| Meconium excretion | 9 (16.1) c | 6 (10.7) | 10 (17.5) | χ2 = 1.154 | 0.562 e |
| Type of meconium | F = 0.329 | 0.999 d | |||
| Thin | 5 (55.6) | 2 (66.7) | 5 (55.6) | ||
| Thick | 4 (44.4) | 1 (33.3) | 4 (44.4) |
Abbreviation: SD, standard deviation.
a Kruskal-Wallis non-parametric test.
b One-way analysis of variance.
c n = 56.
d Fisher’s exact test.
e Chi-square test.
Additionally, the non-parametric Mann-Whitney test revealed that the median time interval from the onset of induction to the onset of labor contractions (P < 0.001), the time interval from the onset of induction to the active phase (P < 0.001), the duration of the active phase of labor (P < 0.001), the duration of the first phase (P < 0.001), the duration of the second phase, and the time from the onset of induction to delivery in the vaginal evening primrose group were significantly shorter than in the oral evening primrose and control groups (P < 0.001). Similarly, the median time interval from the onset of induction to the onset of labor contractions (P < 0.001), the time interval from the onset of induction to the active phase (P < 0.001), the duration of the active phase of labor (P < 0.001), the duration of the first phase (P < 0.001), the duration of the second phase, and the time from the onset of induction to delivery in the oral evening primrose group were significantly shorter than those in the control group (P < 0.001). There were no statistically significant differences among the three studied groups regarding the type of delivery, meconium excretion, meconium quality, and the Apgar score of the newborn (Table 2).
According to the findings of this study, the mean Bishop score before the intervention did not show a statistically significant difference among the three studied groups. However, the differences in mean Bishop scores in the three groups, 2 hours after the intervention and 4 hours after the intervention, were statistically significant. Tukey’s multiple-comparison test demonstrated that the mean Bishop scores two hours after the intervention and four hours after the intervention in the control group were significantly lower than those in the oral evening primrose group (P < 0.001 and P < 0.001, respectively) and the vaginal evening primrose group (P < 0.001 and P < 0.001). Nevertheless, there was no statistically significant difference in the mean Bishop scores 2 hours after the intervention and 4 hours after the intervention between the oral evening primrose group and the vaginal evening primrose group (P = 0.067 and P = 0.756, respectively) (Table 3).
| Variables and Groups | Oral Evening Primrose (n = 57); Mean ± SD (Range) | Vaginal Evening Primrose (n = 56); Mean ± SD (Range) | Control Group (n = 57); Mean ± SD (Range) | F | P-Value a |
|---|---|---|---|---|---|
| Bishop score before intervention | 3.33 ± 1.55 (1 - 6) | 3.70 ± 1.55 (1 - 7) | 3.51 ± 1.51 (1 - 6) | 0.788 | 0.456 b |
| Bishop score 2 hours after intervention | 5.47 ± 1.67 (2 - 9) | 6.28 ± 1.91 (1 - 9) | 4.09 ± 1.61 (1 - 7) | 21.217 | < 0.001 b |
| Bishop score 4 hours after intervention | 7.63 ± 1.59 (3 - 9) | 8.00 ± 1.20 (4 - 9) | 5.18 ± 1.97 (2 - 9) | 28.955 | < 0.001 b |
Abbreviation: SD, standard deviation.
a P < 0.05 as a statistically significant difference.
b One-way analysis of variance.
5. Discussion
In this study, a statistically significant difference was observed in Bishop scores between the oral evening primrose group, vaginal evening primrose group, and the control group, both 2 and 4 hours after induction. The results indicated that the vaginal primrose capsule was more effective in improving the Bishop score. Previous studies have reported similar findings. For instance, one study demonstrated that the use of a 1000 mg evening primrose vaginal capsule after the induction of labor with oxytocin in primiparous women with late pregnancy led to an improvement in the Bishop score in the intervention group (32). Similarly, a study involving women with singleton pregnancies and a gestational age of 39 - 41 weeks revealed that 4 hours after using one capsule of vaginal evening primrose oil during labor induction, the Bishop score significantly improved (33).
In a clinical trial conducted in 2022 on 200 primiparous women with prolonged pregnancies, the use of a single dose of a 1000 mg evening primrose capsule vaginally resulted in an improved Bishop score compared to the placebo (34). Another study in 2019 showed that consuming a 1 000 mg evening primrose capsule at bedtime every night from 38 weeks of pregnancy until delivery led to a higher Bishop score in the intervention group than the placebo group (35). Additionally, an interventional study conducted in the Philippines in 2006 showed that consuming one oral capsule of evening primrose oil daily for one week in women with full-term pregnancies significantly increased the Bishop score and normal delivery rate in the intervention group compared to the placebo group (36). A recent interventional study conducted in 2023 compared the effect of using vaginal misoprostol to a combination of evening primrose and vaginal misoprostol in the treatment of miscarriage abortion among 140 women in Rafsanjan. The results demonstrated that the vaginal evening primrose group exhibited better consistency and dilatation of the cervix (37). The aforementioned findings are consistent with the results of the present study.
However, one study in 2016 reported no statistical difference in Bishop scores between the intervention and placebo groups after daily consumption of 2 oral capsules containing 1 000 mg of oral evening primrose from 40 weeks of pregnancy to 40 weeks and 6 days. This inconsistency with the results of the present study might be attributed to the different administration methods of evening primrose; the previous study used evening primrose without induction (38).
In a clinical trial study conducted in Rafsanjan in 2022, the first intervention group received 2 capsules of 1 000 mg of evening primrose, and the second group received 6 capsules of castor oil concurrently with labor induction. No statistically significant differences were observed between these two groups regarding their effects on cervical ripening and pregnancy outcomes during labor induction (39). It is worth noting that the absence of a control group in this study might have influenced the significance of the Bishop score. Nevertheless, the present study included a control group, and evening primrose was administered differently.
In the present study, the time interval from the onset of induction to the onset of labor contractions, the time interval from the onset of induction to the onset of the active phase, the duration of the active phase of labor, and the time from the onset of induction to delivery in the vaginal evening primrose group were significantly less than those in the oral evening primrose group and the control group. The aforementioned findings are consistent with the results of a study conducted in 2018, which used a 1 000 mg vaginal capsule of evening primrose after labor induction with oxytocin in primiparous women with late pregnancies (25). However, it is important to note that the 2018 study did not compare the results to those of oral evening primrose. It appears that evening primrose can potentially reduce labor duration by improving cervical ripening, and because the mucosal absorption time of vaginal evening primrose capsule is shorter than the oral type, it is more efficient. In a clinical trial study in nulliparous women with post-term pregnancy who received 500 mg vaginal evening primrose capsule and 25 micrograms of sublingual misoprostol, the rate of cervical ripening was significantly higher than that in the control group who took the placebo (40), which is consistent with the present study.
Furthermore, in the present study, the duration of both the first and second phases of labor was significantly shorter in the vaginal evening primrose group than in the oral evening primrose group and the control group. Another study conducted in 2023 demonstrated that the average duration of complete abortion in the group using evening primrose was 8 hours; nevertheless, in the control group, it was 9 hours, indicating a statistically significant difference (37). It is worth noting that the aforementioned study investigated the effect of evening primrose on missed abortion; however, the present study focused on its impact on labor outcomes. Nonetheless, both studies demonstrate the positive influence of vaginal evening primrose on the reduction of pregnancy product expulsion time and the duration of the first and second stages of labor. However, it should be mentioned that in one study, there was no significant difference in the duration of the first and second phases of labor (35). In the aforementioned study, pregnant mothers were prescribed one 1000 mg vaginal capsule every night starting from the 38th week until delivery. However, in the present study, two 1000 mg vaginal capsules were prescribed to be used concurrently with induction. Therefore, it can be suggested that the shorter the interval between the use of evening primrose and delivery, the more pronounced the effects on the length of labor phases might be.
A 2019 study investigated the impact of evening primrose oil capsules on cervical preparation and the onset of labor pain. The intervention group participants had 2 soft primrose capsules placed in the posterior choledosac. The control group received placebo capsules. The results indicated no difference in the duration of the first and second stages of labor (40), which contrasts with the findings of the present study. This discrepancy is probably due to the early intervention at 37 weeks and the unpreparedness of the cervix.
In the current study, there were no statistically significant differences in meconium excretion and Apgar scores at the first and fifth minutes. Some previous studies (26, 35, 36) also reported no statistically significant differences in meconium excretion and Apgar scores between the two groups, aligning with the results of the present study. It appears that the use of evening primrose does not lead to fetal distress.
One of the strengths of this project is the absence of prior comparisons between the use of vaginal evening primrose and oral evening primrose concurrent with induction. However, a limitation of this study was the impact of the coronavirus disease 2019 (COVID-19) pandemic. Since some variables were extracted from medical records, there might have been bias in measurements conducted by different individuals.
5.1. Conclusion
Based on the results of this study, it appears that compared to the control group, which did not receive any specific medication related to evening primrose and only underwent induction, both oral and vaginal evening primrose capsules, particularly the vaginal type, can influence cervical ripening and pregnancy outcomes during labor induction. Therefore, the clinical use of vaginal evening primrose might contribute to a reduction in the duration of labor and yield positive results. Therefore, future studies could explore the effects of different doses of oral and vaginal evening primrose without induction during delivery to better evaluate the actual impact of this medicinal plant on labor.