1. Background
The worldwide spread of SARS-CoV-2 and thousands of deaths caused by the coronavirus disease 2019 (COVID-19) led the World Health Organization (WHO) to declare it a pandemic on March 12, 2020 (1). Following on, many vaccines were quickly manufactured in a way that the first COVID-19 vaccine entered human clinical trials on March 16, 2020 (2), and before mid-2021, approximately 3 billion various COVID-19 vaccines were administered worldwide, including Sinopharm, Covax, Moderna, PFIZER, and AstraZeneca (3). Women form a major part of the world's population, and one of the crucial aspects of studying women's physical and reproductive health is their regular menstrual cycle (4). The characteristics of the menstrual cycle are increasingly recognized as "vital signs" (4), and irregular and longer menstrual cycles increase the risk of dying before the age of 70, as well as the risk of contracting metabolic diseases such as diabetic mellitus and dyslipidemia in women (5, 6).
Therefore, menstrual disorders are a major challenge for health care systems, especially when the focus is on their effects on the daily activities of women (5). One of the major issues in COVID-19 vaccination was the concern regarding the possible connection between COVID-19 vaccination and the creation of unnatural menstrual cycles (7). This idea was strengthened when several reports were issued on menstrual irregularities and vaginal bleeding after COVID-19 vaccination in social media, vaccine adverse event reporting systems (VAERS), vaccine monitoring systems in Britain and Norway, and online surveys (8). In a study conducted in MENA (Middle East and North Africa) in 2022 on 2269 women in the age range of 14 to 54 years, the results showed that 66.3% of the participants had complaints of menstrual disorders after vaccination against COVID-19, especially after the first dose (9). In another study carried out in Italy in 2022 through publishing online questionnaires in Italian on internet-based platforms, such as Facebook and Twitter, 50 - 60% of women of reproductive age had reported irregularities in their menstrual cycles after injection of the first dose, while 60 - 70% of women had the same issues after injection of the second dose, regardless of the type of vaccine (10). These results lead to doubt and fear of the effects of COVID-19 vaccination on fertility (11, 12).
The menstrual cycle is the result of complex interactions between different tissues, hormones, and organ systems, and physiological and pathological variables, including viral infections and changes in lifestyle, can cause changes to it (13). COVID-19 is considered a pro-inflammatory disease that causes cytokine storms, which lead to immune system exhaustion (13). The inflammatory response has a role in tissue repair and angiogenesis and the destruction, regeneration, and proliferation of the endometrium (13). It has been reported that SARS-CoV-2 infection, regardless of vaccination status, can cause changes to the menstrual cycle (13). On the other hand, stressful events or an exaggerated immunologic response, such as those caused by immunization, can temporarily affect the menstrual cycle by affecting the hypothalamic-pituitary-gonadal (HPG) axis and follicular maturation during the follicular phase (9, 13).
Unfortunately, clinical trials that address the adverse reactions of COVID-19 vaccination do not provide specific data on menstrual cycle changes after vaccination (13). Even though the Medicines and Healthcare Products Regulatory Agency (MHRA) of Britain has found 48 488 menstrual disorders-related reactions after COVID-19 vaccination until January 2022 (13), they have reported that they do not support the monitoring reports concerned with the relationship between changes in menstrual periods and COVID-19 vaccines. However, they have recently suggested the existence of a potential relationship between the two and requested that more research be performed (10).
2. Objectives
Therefore, considering the limited information on the effects of COVID-19 vaccination on women's menstruation, this study was carried out with the aim of investigating the prevalence of menstrual disorders in women vaccinated against COVID-19 and its relation with the type of administered vaccine.
3. Methods
3.1. Study Design and Participants
This study was a descriptive-analytical cross-sectional study carried out in 2022 with the participation of 400 women of reproductive age (15 - 49 years) who had received at least 2 doses of a COVID-19 vaccine (regardless of its type). The participants were chosen from 4 urban health centers in the city of Asadabad of Hamedan Province in Iran. Inclusion criteria were comprised of women who were in the reproductive age who had received 2 doses of COVID-19 vaccine at least 6 months prior to the study, remembered their menstrual date before and after vaccination, and were aware of their vaccination dates. Exclusion criteria were comprised of lack of consent for participation in the study, suffering from any disease known to affect the menstrual cycle, having a history of irregular menstrual cycle prior to vaccination, menopause, pregnancy 3 months after vaccination, having a history of recent delivery, and filling incomplete questionnaires.
3.2. Sampling Method
Participants were chosen from 4 urban health centers in the city of Asadabad using the convenience sampling method.
3.3. Sample Size
The required sample size in this research was calculated to be 400 individuals based on a previous study (10), and by considering 90% power and significance level of 0.01 (w [effect size] = 0.26), utilizing PWR software package in R v. 3.6.1 software program and by considering 10% attrition rate.
3.4. Tools
The tool used in this research was a demographics questionnaire for collecting information about age, marital status, number of children, level of education, occupation, etc. Furthermore, a researcher-made questionnaire including 27 questions was used for assessing the type and number of received vaccines and changes in the menstrual cycle (including changes in its length as well as quantity and quality before, during, and after vaccination), and whether the recipient had experienced mental or psychological changes after COVID-19 vaccination. Questions were designed based on similar research studies and were confirmed by 10 faculty members of Asadabad University of Medical Sciences. Internal consistency of the questionnaire was obtained using Cronbach's alpha coefficient of 0.86.
3.5. Statistical Analysis
The data from collected questionnaires was fed into SPSS v. 24 (IBM Corp., Armonk, NY, USA). Descriptive statistics, including frequency and percentage, were used for data analysis. For assessing the aims of the research, chi-square and Fisher's exact tests were used with a significance level of 0.05.
3.6. Ethical Considerations
This study received the approval of the Ethics Committee of the Asadabad Faculty of Medical Sciences under the ethics code IR.ASAUMS.REC.1401.011, and the procedure was in accordance with the ethical principles of the Declaration of Helsinki. The participants were thoroughly informed of the aims of the study, and written consent was obtained from each participant knowingly. In addition, they were assured that their information would remain confidential and only in the possession of the researcher and that the results would be published anonymously.
4. Results
In this study, 400 women of reproductive age (15 - 49 years) who had received at least 2 doses of COVID-19 vaccine (regardless of its type) were included. Most participants were married, were homemakers, had university-level education, and were within the age range of 30 to 39. The most common type of contraception among the participants was the withdrawal method (Table 1). The most frequently used type of vaccine in all 3 doses was Sinopharm (Table 2). After administration of the first dose, more than one-third of the participants experienced menstrual disorders, and this number was increased to approximately half the participants after administration of the second and third doses (Table 3). Reported menstrual disorders included changes in the regularity of menstruation, volume of bleeding, or length of the menstrual cycle. A significant relation was observed between menstrual disorders and level of education in the third dose of the vaccine, and individuals with academic education suffered from more disorders (Table 1).
| Variables | No. (%) | Menstrual Disorders | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| After the First Dose | After the Second Dose | After the Third Dose | ||||||||
| Yes | No | Chi-square Test | Yes | No | Chi-square Test | Yes | No. a | Chi-square Test | ||
| Age (y) | ||||||||||
| 15 - 19 | 30 (7.5) | 12 | 18 | 4.736; P = 0.192 | 13 | 17 | 7.474; P = 0.058 | 4 | 26 | 2.608; P = 0.456 |
| 20 - 29 | 149 (37.3) | 63 | 86 | 73 | 76 | 40 | 109 | |||
| 30 - 39 | 150 (37.5) | 46 | 104 | 51 | 99 | 35 | 115 | |||
| 40 - 49 | 71 (17.8) | 24 | 47 | 33 | 38 | 18 | 53 | |||
| Education level | ||||||||||
| High school | 192 (48) | 62 | 130 | 3.330; P = 0.343 | 78 | 114 | 1.287; P = 0.732 | 33 | 159 | 11.080; P = 0.011 |
| Academic | 208 (52) | 124 | 82 | 91 | 115 | 63 | 143 | |||
| Job | ||||||||||
| Homemaker | 160 (40) | 58 | 102 | 2.739; P = 0.343 | 70 | 90 | 0.176; P = 0.981 | 28 | 132 | 7.031; P = 0.071 |
| Employed | 149 (37.2) | 49 | 100 | 62 | 87 | 42 | 107 | |||
| Student | 91 (22.8) | 38 | 53 | 38 | 53 | 27 | 64 | |||
| Variables | No. (%) | Yes | No | Fisher Exact Test | Yes | No | Fisher Exact Test | Yes | No. a | Fisher Exact Test |
| Contraceptive method | 8.797; P = 0.097 | 8.121; P = 0.126 | 11.424; P = 0.034 | |||||||
| LD, HD, or triphasic tablets | 33 (8.3) | 17 | 16 | 18 | 15 | 13 | 20 | |||
| Three-month ampoule | 1 (0.3) | 1 | 0 | 1 | 0 | 1 | 0 | |||
| Condom | 39 (9.8) | 15 | 24 | 18 | 21 | 8 | 31 | |||
| Withdrawal | 126 (31.5) | 38 | 88 | 58 | 68 | 22 | 104 | |||
| IUD | 18 (4.5) | 4 | 14 | 4 | 14 | 3 | 15 | |||
| None | 183 (45.8) | 70 | 113 | 71 | 112 | 50 | 133 | |||
| Marital status | 1.718; P = 0.408 | 1.430; P = 0.492 | 1.831; P = 0.385 | |||||||
| Married | 248 (62) | 85 | 163 | 106 | 142 | 55 | 193 | |||
| Single | 142 (35.5) | 55 | 87 | 58 | 84 | 40 | 102 | |||
| Divorced or widowed | 10 (2.5) | 5 | 5 | 6 | 4 | 2 | 8 | |||
a It includes people who either did not have a menstrual disorder or did not receive the third dose.
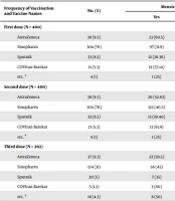
| Frequency of Vaccination and Vaccine Names | No. (%) | Menstrual Disorder; No. (%) | Chi-square Test | |
|---|---|---|---|---|
| Yes | No | |||
| First dose (N = 400) | 16.357; P = 0.003 | |||
| AstraZeneca | 38 (9.5) | 23 (60.5) | 15 (39.5) | |
| Sinopharm | 304 (76) | 97 (31.9) | 207 (68.1) | |
| Sputnik | 33 (8.3) | 12 (36.36) | 21 (63.64) | |
| COVIran Barekat | 21 (5.3) | 12 (57.14) | 9 (42.86) | |
| etc. a | 4 (1) | 1 (25) | 3 (75) | |
| Second dose (N = 400) | 5.981; P = 0.201 | |||
| AstraZeneca | 38 (9.5) | 20 (52.63) | 18 (47.37) | |
| Sinopharm | 304 (76) | 123 (40.5) | 181 (59.5) | |
| Sputnik | 33 (8.3) | 13 (39.40) | 20 (60.6) | |
| COVIran Barekat | 21 (5.3) | 13 (61.9) | 8 (38.1) | |
| etc. a | 4 (1) | 1 (25) | 3 (75) | |
| Third dose (N = 202) | 124.494; P ≤ 0.001 | |||
| AstraZeneca | 37 (9.3) | 22 (59.5) | 15 (40.5) | |
| Sinopharm | 124 (31) | 56 (45) | 68 (55) | |
| Sputnik | 20 (5) | 7 (35) | 13 (65) | |
| COVIran Barekat | 5 (1.3) | 3 (60) | 2 (40) | |
| etc. a | 16 (4.3) | 8 (50) | 8 (50) | |
a etc: Bharat, PastoCovac, Cov Pars.
| Frequency of Vaccination | Menstrual Disorders; No. (%) | Chi-square Test | |
|---|---|---|---|
| Yes | No | ||
| First dose (N = 400) | 145 (36.3) | 255 (63.7) | 30.523; P ≤ 0.001 |
| Second dose (N = 400) | 170 (42.5) | 230 (57.5) | |
| Third dose (N = 202) | 97 (48.1) | 105 (51.99) | |
A significant relation was observed between menstrual disorders and type of contraception in the third dose of the vaccine, and most disorders in the third dose were related to individuals who did not use any method of contraception (Table 1). No significant relationship was found between menstrual disorders and occupation, age, and marital status in any of the doses (Table 1).
The results showed that the type of vaccine administered in the first and third doses had a relationship with causing menstrual disorders. The most menstrual disorders were in the first dose, with 60.5% being related to the AstraZeneca vaccine, while in the third dose, 60% were related to the COVIran Barekat vaccine (Table 2).
All the participants received the first and second doses of the vaccine, but only 202 individuals received a third dose (Table 3). Significant relationships were observed between the number of vaccination doses and menstrual disorders (Table 3).
In addition, the results showed a significant relationship between the frequency of menstrual disorders and mental disorders (bad-temperedness, sadness, and depression) after administration of the vaccine in all 3 doses (Table 4).
| Frequency of Vaccination and Menstrual Disorder | Mental Disorder | Chi-square Test | |
|---|---|---|---|
| Yes | No | ||
| First dose (N = 400) | 15.565; P ≤ 0.001 | ||
| Yes | 51 | 94 | |
| No | 45 | 210 | |
| Second dose (N = 400) | 38.243; P ≤ 0.001 | ||
| Yes | 80 | 90 | |
| No | 42 | 188 | |
| Third dose (N = 202) | 147.967; P ≤ 0.001 | ||
| Yes | 43 | 54 | |
| No | 18 | 87 | |
5. Discussion
The current study was performed with the aim of investigating the prevalence of menstrual disorders after COVID-19 vaccination and their relationship with the type of administered vaccine in the city of Asadabad of Hamedan Province in Iran. The results revealed that the participants suffered from menstrual disorders (irregularity, bleeding, or length of menstruation) after receiving COVID-19 vaccines, and these disorders were intensified by increasing the number of vaccine administration. A similar study performed in MENA on 2269 women in the age range of 14 - 54 years showed that 66.3% of the study participants had suffered from menstrual disorders after vaccination against COVID-19, especially after the first dose (9). This difference in the increase of frequency of menstrual disorders after vaccine administration can be due to differences in the types of administered vaccines in that study. Furthermore, in another study carried out by publishing online questionnaires in Italian on internet-based platforms such as Facebook and Twitter, 50 - 60% of the participants had reported irregularities in their menstrual cycles, regardless of the type of vaccine (10). Additionally, in a systematic review study carried out in 2022, 52.5% of women had some type of menstrual disorder after vaccination (14).
The menstrual cycle includes complex interactions between different tissues, hormones, and organ systems, and physiological and pathological variables, including viral infections and changes in lifestyle, can cause changes to it (13). COVID-19 is considered a pro-inflammatory disease that causes cytokine storms, which lead to immune system exhaustion (13). The inflammatory response has a role in tissue repair, angiogenesis, destruction, regeneration, and proliferation of the endometrium (13). It has been reported that SARS-CoV-2 infection, regardless of vaccination status, can cause changes to the menstrual cycle (13). On the other hand, stressful events or an exaggerated immunologic response, such as those caused by immunization, can temporarily affect the menstrual cycle by affecting the HPG axis and follicular maturation during the follicular phase (9, 13). In this study, the prevalence of menstrual disorders was increased by the rise in the number of vaccinations, which corresponds to the results of a similar study (10).
The findings of this study revealed that the type of administered vaccine in the first and third doses had a relationship with causing menstrual disorders. However, since the number of vaccine recipients of every type of vaccine was not the same in this study, this finding cannot be extrapolated, and more investigation is required. With regard to the lack of relationship between the type of vaccine and frequency of menstrual disorder in the second dose, some studies have pointed out the self-limiting aspect of menstrual disorders after COVID-19 vaccination (9), which might result in such disorders not being repeated after the second dose. Results of a similar cross-sectional study carried out in the MENA region, in which 2 types of their administered vaccines were similar to the ones injected in this work (Sinopharm and AstraZeneca), showed that the type of vaccine is not related to menstrual disorders, which is not consistent with the present study (9). This observation can be due to differences in the studied society and race. In addition, in a study performed on 721 participants in India with the aim of investigating menstrual disorders after COVID-19 vaccine administration (Covaxin and Covishield), it was revealed that these disorders were considerably increased after Covaxin administration (15).
There was a significant relationship between menstrual disorders and the level of education of women after administration of the third dose, and women with university-level education suffered from more disorders compared to women with high school diplomas or lower levels of education. This can be due to differences in self-care behaviors in various levels of society as well as health literacy levels and awareness with regard to symptoms of menstrual disorders in the studied women. Moreover, there was no relationship between menstrual disorders and the occupation and age of the women in any of the doses. During our research, no similar study was found that had investigated the relationship between menstrual disorders and occupation as well as level of education. Moreover, similar studies have not reported a significant relationship between age and menstrual disorders (9, 16). Furthermore, there was no considerable relationship between menstrual disorders and type of contraception in the first and second doses of vaccine administration, but this connection was significant in the third dose, and most menstrual disorders in the third dose were among individuals who were not using any method of contraception. Considering the fact that some of the contraception methods, especially hormonal methods, are among the effective treatments for menstrual disorders such as dysmenorrhea, menorrhagia, and endometriosis, it is possible that the occurrence of menstrual disorders is falsely hidden in the studied women who used one method of contraception (17).
In this study, there was a significant relationship between the frequency of menstrual disorders and experiencing mental disorders (bad-temperedness, sadness, and depression) after administration of the vaccine in all 3 doses. In a prospective study performed in Arizona, United States, with 545 women participants, 43.5% experienced mood swings in premenstrual periods after receiving COVID-19 vaccines (16). The social anxiety caused by the COVID-19 pandemic, lack of trust in the safety of vaccination among the public, and negative thoughts can lead to stress, anxiety, and mental disorders in people after vaccination (18). The time of the menstrual cycle is regulated by the HPG axis, which can be affected by life, environment, and health stressors, and vaccination is indeed one of the environmental stressors (19-22). Stress factors can temporarily cause menstrual disorders by affecting this axis (23-25). This conjecture that vaccines cause menstrual disorders also exists with regard to many other vaccines, including measles, mumps, rubella, hepatitis A and B, influenza, etc. (26, 27), and only different studies investigating the various effects of vaccination can reject or confirm such speculations.
The strength of this study is assessing menstrual disorders in all 3 doses of vaccine, while many other studies only assess these disorders in a general sense. Moreover, this study investigated the disorders in Iran, and considering the low number of similar studies, this work can be effective in the final conclusion of investigating the prevalence of menstrual disorders throughout the world after COVID-19 vaccination. However, there were certain limitations in this work. The self-reporting aspect of the questionnaires, non-random sampling, lack of existence of a control group, not investigating the duration of menstrual disorders after administration of vaccines, and the long interval between administration of vaccine doses in terms of their effects on menstrual cycles of women were among the limitations of this study. Since this was a cross-sectional study, it cannot be firmly stated whether vaccine administration can result in menstrual irregularities or not.
5.1. Conclusions
Some instances of menstrual disorders (changes in regularity, duration, and volume of bleeding) were observed in the women of this study after COVID-19 vaccination, which were intensified by increasing the number of vaccine administration. These irregularities can affect daily life activities and, as a result, can disrupt the normal flow of life, including absence from the workplace or place of education, and can lessen the quality of women's lives. However, considering the limitations of this study, it cannot be said with certainty whether the administration of the COVID-19 vaccine results in menstrual irregularities or not. More prospective studies should be carried out to identify the connection between menstrual cycle irregularities and the continuation of such disorders with various COVID-19 vaccines. Moreover, it is recommended that case-control studies should be applied in the future in order to determine the duration of the effects of vaccines.