1. Background
In December 2019, cases of acute respiratory failure abruptly increased in Wuhan, China. This sudden rise led to the identification of Severe Acute Respiratory Syndrome Coronavirus 2, or SARS-CoV-2, as the novel pathogen responsible for the surge. The disease was officially classified as novel coronavirus disease (COVID-19) by the World Health Organization (WHO). On March 11, 2020, WHO declared this condition a global pandemic (1, 2).
Based on various studies, it has been observed that the novel coronavirus can infect individuals across all age groups, from newborns to the elderly. However, certain groups are more susceptible to the infection, with pregnant women representing one such vulnerable demographic. Compared to the general population, pregnant women exhibit heightened sensitivity to infectious diseases, especially respiratory illnesses and severe pneumonia (3).
Physiological adaptations during pregnancy, such as a rise in the diaphragm level, increased oxygen consumption, and respiratory tract mucosa edema, lead to decreased tolerance to hypoxia (4). A decline in the acute immune response to inflammation occurs as a protective mechanism against fetal rejection. These alterations in the body's cardiopulmonary immune system make pregnant mothers more prone to viruses (5). Additionally, both mechanical and biochemical factors influence gas exchange and pulmonary function throughout pregnancy, such as the decrease in residual volume. During an infectious disease epidemic, these factors make pregnant mothers, their fetuses, and newborns more vulnerable. Furthermore, in the context of an infectious disease outbreak, particular emphasis should be placed on newborns, as infected infants may either exhibit asymptomatic conditions or present with mild or severe symptoms (6).
There are significant concerns about the disease's prevention and management in pregnant women, coupled with the potential risk of vertical transmission (7). Few studies have investigated the repercussions of the coronavirus outbreak on Iranian mothers who tested positive for SARS-CoV-2 and their newborns since its onset.
2. Objectives
The objective of the current study was to assess the outcomes of pregnant patients admitted to hospitals and medical facilities in Sistan and Baluchestan province, Iran during 2019 - 2020, who had laboratory-confirmed SARS-CoV-2 infection.
3. Methods
The aim of this retrospective study was to investigate the frequency of maternal and fetal outcomes in pregnant mothers with COVID-19 within hospitals affiliated with Zahedan University of Medical Sciences in 2019 - 2020. All pregnant women who presented to the hospitals within a six-month timeframe (October to March) were evaluated. During this period, about 150 mothers suspected of coronavirus infection were identified, with 100 of them confirmed through a positive RT-PCR test and subsequently enrolled in the study. Additionally, 50 healthy pregnant women who sought care from these centers and were either asymptomatic or had a negative COVID-19 test result during their hospital stay were selected as the control group. The criteria for exclusion from the research included the unwillingness of pregnant mothers and personnel to participate in the study, as well as mothers who declined to undergo COVID-19 testing.
Data collection involved using a form created based on the medical history of pregnant mothers with COVID-19 and information extracted from patients' records, encompassing demographic and medical details.
Following the approval and confirmation of the thesis title, obtaining the ethical code from the ethics committee of Zahedan University of Medical Sciences, and acquiring a legal introduction letter from the university, we proceeded to the designated medical centers. Using the distribution map of healthcare facilities, including maternity hospitals and comprehensive health service centers in Sistan and Baluchestan province, specifically in Zahedan, Khash, Saravan, and Mirjaveh cities, we randomly selected five centers from diverse regions. In adherence to COVID-19 social distancing guidelines, we conducted phone calls to individuals meeting the entry criteria. We provided necessary explanations and obtained their informed consent to participate in the study.
Basic information was extracted and analyzed from the Sib system, an integrated health system containing Iranian electronic health record information, and patient records. Additionally, by conducting on-site visits to the selected medical centers, pregnant mothers meeting the study criteria were identified. Following the registration of patients' information, a cohort of 100 pregnant mothers with COVID-19 was selected and diligently followed up.
This research employed both descriptive and inferential statistical methods. In the descriptive section, statistical characteristics of the sample were outlined, and frequency distribution tables were used. Reports on qualitative variables were presented as percentages. Data analysis was conducted using the chi-square test with SPSS software version 25. The significance threshold for the tests was set at P < 0.05.
4. Results
A total of 150 pregnant women (100 cases and 50 controls) participated in the study. The average age of pregnant women with COVID-19 was 31.40 ± 5.51 years, while that of healthy pregnant women was 30.77 ± 5.86 years. The average number of pregnancies in the case and control groups was 2.26 ± 2.00 and 2.68 ± 1.96, respectively. There were no significant differences between the cases and controls regarding age and the average number of pregnancies (P > 0.05). All women in both groups were carrying singleton pregnancies.
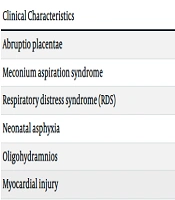
As depicted in Table 1, preterm birth was the most prevalent complication among pregnant women with COVID-19, with a frequency of 37%. Other notable complications included respiratory distress syndrome (21%), neonatal asphyxia (16%), meconium aspiration syndrome (15%), premature rupture of membranes (13%), stillbirth (10%), and oligohydramnios (6%). Additionally, 30% of cases underwent cesarean section. Less than four percent of pregnant women experienced abruptio placentae (3%), cardiorespiratory arrest (2%), and abortion (2%). The maternal mortality rate was 8%.
| Clinical Characteristics | Yes (%) | No (%) |
|---|---|---|
| Abruptio placentae | 3 | 97 |
| Meconium aspiration syndrome | 15 | 85 |
| Respiratory distress syndrome (RDS) | 21 | 79 |
| Neonatal asphyxia | 16 | 84 |
| Oligohydramnios | 6 | 94 |
| Myocardial injury | 2 | 98 |
| Maternal mortality | 8 | 92 |
| Preterm birth | 37 | 63 |
| Stillbirth | 10 | 90 |
| Premature Rupture of Membrane (PROM) | 13 | 87 |
| Abortion | 2 | 98 |
| Cesarean section | 30 | 70 |
Table 2 shows the comparison of neonatal outcomes between pregnant women with COVID-19 (n = 100) and healthy mothers (n = 50). The comparative analysis revealed significant differences between the two groups regarding preterm birth (37% vs. 14%, P = 0.004) and premature rupture of membranes (13% vs. 2%, P = 0.035).
| Clinical Characteristics | Infected Mothers | Healthy Mothers | P-Value |
|---|---|---|---|
| Abruptio placentae | |||
| Yes | 3 | 6 | 0.401 |
| No | 97 | 94 | |
| Meconium aspiration syndrome | |||
| Yes | 15 | 22 | 0.360 |
| No | 85 | 78 | |
| Cardiorespiratory arrest | |||
| Yes | 2 | 0 | 0.553 |
| No | 98 | 100 | |
| Maternal mortality | |||
| Yes | 8 | 0 | 0.052 |
| No | 92 | 100 | |
| Preterm birth | |||
| Yes | 37 | 14 | 0.004 |
| No | 63 | 86 | |
| Premature Rupture of Membrane (PROM) | |||
| Yes | 13 | 2 | 0.035 |
| No | 87 | 98 | |
| Respiratory distress syndrome (RDS) | |||
| Yes | 21 | 24 | 0.681 |
| No | 79 | 76 | |
| Stillbirth | |||
| Yes | 10 | 2 | 0.101 |
| No | 90 | 98 | |
| neonatal asphyxia | |||
| Yes | 16 | 10 | 0.455 |
| No | 84 | 90 | |
| Oligohydramnios | |||
| Yes | 6 | 2 | 0.425 |
| No | 94 | 98 | |
| Abortion | |||
| Yes | 2 | 0 | 0.553 |
| No | 98 | 100 | |
| Cesarean section | |||
| Yes | 30 | 38 | 0.359 |
| No | 70 | 62 |
a Values are expressed as (%).
The signs and symptoms observed in pregnant women infected with COVID-19 are illustrated in Table 3. While 24% of cases were asymptomatic, shortness of breath was identified in 17% of women with COVID-19. Cough, shortness of breath+cough, fever+cough, and fever were observed in 16%, 15%, 14%, and 5% of cases, respectively.
| Signs and Symptoms | Percent |
|---|---|
| Fever+cough | 14 |
| shortness of breath+cough | 15 |
| Shortness of breath | 17 |
| Cough | 16 |
| Fever | 5 |
| (Asymptotic) with no sign | 24 |
| Fever+chills | 1 |
| Cough+shortness of breath+fever | 4 |
| Cough+fever and chills | 1 |
| Cough+shortness of breath+chest pain | 1 |
| Cough+shortness of breath+fever and chills | 1 |
| Cough+shortness of breath+fever+chest pain | 1 |
5. Discussion
In this study, our findings regarding the maternal and neonatal outcomes in pregnancies positive for COVID-19 indicated that among mothers with COVID-19, 37% experienced complications associated with premature birth, 21% had complications related to acute respiratory distress syndrome, and 10% experienced complications resulting in stillbirth. Notably, 24% of mothers were asymptomatic, and among other complications, shortness of breath was the most common (17%). A caesarean section was performed in 30% of mothers with COVID-19, and only 2% of the cases experienced a miscarriage. It is noteworthy that the majority of mothers with COVID-19 in this study did not experience complications such as placental abruption, meconium aspiration in the fetus, cardiorespiratory arrest, premature rupture of membranes, asphyxia, and oligohydramnios in the fetus. The observed maternal mortality rate was 8%. Additionally, a statistically significant difference was found between mothers with COVID-19 and healthy mothers in the frequency of premature birth and premature rupture of membranes.
During pregnancy, immunosuppressive responses and physiological adaptive changes, including an increased hemidiaphragm, elevated oxygen consumption, and respiratory mucosal edema, contribute to hypoxia in pregnant women. These factors make pregnant women more susceptible to respiratory tract infections and severe pneumonia (8).
There is now widespread acknowledgment that the respiratory symptoms of COVID-19 exhibit considerable heterogeneity, ranging from minimal symptoms to pronounced hypoxia leading to acute respiratory distress syndrome (ARDS). The reported timeframe between the onset of symptoms and the development of ARDS is approximately 9 days, highlighting the potential for rapid progression of respiratory symptoms. It's important to remember that COVID-19 can be fatal. Therefore, in pregnant women, monitoring for the development of symptoms and signs of COVID-19 should be taken seriously (9).
There is a lack of knowledge about how pregnancy affects COVID-19 and vice versa. Compared to their non-pregnant counterparts, pregnant women with COVID-19 often experience more severe illness, as evidenced by increased mortality rates, higher rates of intensive care unit admissions, and the need for supplemental oxygen and ventilation (10). According to a recent meta-analysis by Dubey et al., 27% of pregnant individuals infected with COVID-19 experienced adverse pregnancy outcomes, such as vascular malperfusion of the fetus, premature delivery, and premature rupture of the fetal membrane (11).
In research conducted by Allotey et al., results showed that pregnant women with COVID-19 who were admitted to the hospital for any reason manifested a lower incidence of symptoms such as fever, cough, dyspnoea, and myalgia. However, compared to non-pregnant women of reproductive age, pregnant women with COVID-19 were more prone to admission to the intensive care unit or requiring invasive ventilation. Moreover, delivering preterm is more likely in pregnant women with COVID-19 compared to those without COVID-19. Additionally, the risk of maternal death and requiring intensive care unit admission is higher in pregnant women with COVID-19 (12). Furthermore, Sacinti et al. found that in Turkey, the rate of miscarriages during the COVID-19 pandemic in 2020 was higher than in prior years (13).
Given the limited studies conducted in Iran on fetal outcomes in mothers with COVID-19, and recognizing the importance of managing and controlling conditions to prevent complications for both mothers and their neonates, accurate information is crucial across all regions of Iran. Thus, to investigate the frequency of fetal outcomes in mothers infected with COVID-19 at hospitals affiliated with Zahedan University of Medical Sciences in 2019 and 2020, this study was conducted.
This study found that 37% of mothers with COVID-19 experienced preterm birth, which was higher than reported in previous studies. In the study conducted by Martinez-Perez et al., a total of 1009 pregnancies were screened, comprising 246 cases with a positive status for SARS-CoV-2 and 763 mothers testing negative. Their findings indicated that SARS-CoV-2 infection elevated the odds of preterm birth (13.8% vs. 6.7%, P = 0.002) and iatrogenic preterm delivery (4.9% vs. 1.3%, P = 0.001). However, the incidence of spontaneous preterm deliveries did not show a statistically significant difference (6.1% vs. 4.7%). Furthermore, the study revealed an increased risk of premature rupture of membranes at term (15.8% vs. 9.8%, P = 0.013) and higher rates of neonatal intensive care unit admissions among mothers with positive SARS-CoV-2 status (9.3% vs. 2.4%, P < 0.001) (14). An additional estimation conducted in 2020 revealed that, compared to mothers with asymptomatic COVID-19, preterm labor occurred three times more commonly in symptomatic mothers (8).
In the present study, the frequency of acute respiratory distress syndrome (ARDS) in fetuses of mothers infected with COVID-19 was 21%. In contrast, the cross-sectional study carried out by Vimercati et al. on 122 pregnant women with COVID-19 infection found the frequency of respiratory distress syndrome to be 5.6% (15). Additionally, although none of the neonates tested positive for COVID-19 infection, the Neonatal Intensive Care Unit (NICU) admission rate was 10.4%.
Our results regarding the frequency of premature rupture of membranes (PROM) in mothers with COVID-19 showed that 87% of affected mothers in this study did not experience the complication of premature rupture of membranes. In Azh et al.'s 2019 study on 133 pregnant women with COVID-19 in Qazvin province, Iran, the rate of premature rupture of membranes was reported as 2.3%, which was lower than our findings (13%) (16).
Moreover, 10% of the COVID-19 mothers in this study experienced a stillbirth. Lyu et al. examined a total of 191,403 pregnancies involving 190,738 individuals who gave birth between March 2020 and May 2021. The main emphasis was on stillbirths occurring at least 20 weeks into pregnancy. In early pregnancy, there were 2,342 (1.3%) pregnancies with COVID-19, 2,075 (1.2%) in mid-pregnancy, and 12,697 (6.9%) in the third trimester. The findings revealed that pregnant women who were infected with COVID-19 in the early and mid-stages of their pregnancy had a higher risk of stillbirth. Infection cases that occurred during the third trimester or at any point prior to delivery did not exhibit this risk. These results suggest that the fetus may be susceptible to SARS-CoV-2 infection during the early stages of pregnancy. Other variables associated with stillbirth in various trimesters included older age, black ethnicity, hypertension, acute respiratory distress syndrome or acute respiratory failure, and placental abruption (17).
The mortality rate for mothers who contracted COVID-19 in our study was 8%, which is significantly higher than in previous research. For example, the study by Asalkar et al. reported a maternal mortality rate of 1.03%, indicating that out of 871 COVID-19 cases diagnosed during pregnancy, nine maternal deaths occurred (18).
Our assessment of the frequency of placental abruption in mothers with COVID-19 revealed that the majority of participants (97%) did not experience this complication. However, Rodriguez-Diaz et al. suggested a relationship between SARS-CoV-2 infection and an increased risk of placental abruption (19).
Furthermore, our results demonstrated that the complication of meconium aspiration in the fetus was absent in 85% of mothers with COVID-19. While 2% of mothers in our study experienced cardiorespiratory arrest, a previous study reported a higher rate (9.7%) (20).
The results obtained from assessing the incidence of neonatal asphyxia in mothers with COVID-19 indicated that the majority of mothers in this study (84%) did not experience neonatal asphyxia. Similar findings have been reported in previous studies. In a study conducted by Taghavi et al. involving 55 pregnant women infected with COVID-19 and an equal number of control pregnant women, obstetric, maternal, and neonatal outcomes were assessed. The study found no significant differences between the two groups in terms of birth weight, Apgar score, premature rupture of membranes, postpartum hemorrhage, newborn asphyxia rate, and delivery mode (21).
According to reports, oligohydramnios was significantly more prevalent in pregnant women infected with COVID-19 compared to those with COVID-negative status. In our study, oligohydramnios was present in the fetus in 4% of the mothers with COVID-19 (22).
In our study, only 2% of mothers infected with COVID-19 experienced miscarriage, which is consistent with previous reports. A recent study by Hajialakbari et al. revealed that the occurrence of miscarriage in pregnant women infected with SARS-CoV-2 was 3.9% (23). According to the ethnicity-based stratified analysis, the incidence of miscarriage was 2.1% in Caucasians, West Asians, and Europeans, compared to 6.3% in infected pregnant Asian women. Furthermore, in this study, 30% of COVID-19 mothers underwent cesarean sections, which aligns with the findings of a 2020 meta-analysis by Soheili et al. (24).
The examination of the most common symptoms among mothers with COVID-19 revealed that the highest prevalence was among asymptomatic mothers (24%), and the most prevalent symptom was shortness of breath (17%). In a systematic review of SARS-CoV-2 infection and pregnancy, including 17 studies (2567 pregnancies), the most commonly reported clinical symptoms were fever (63.3%), cough (71.4%), and dyspnea (34.4%) (25).
The comparison of neonatal outcomes between mothers with COVID-19 and healthy mothers revealed a significantly higher incidence of premature birth and premature rupture of membranes in the COVID-19 group. This finding aligns with a report presented by Azh et al. in 2019 from Qazvin province, Iran (16).
Various factors may contribute to the observed differences in study outcomes. Firstly, limited research has been conducted on the effects of COVID-19 on pregnant mothers and their infants across diverse countries, with a notable gap in studies conducted in Iran. Secondly, variations in health and treatment protocols for the prevention and management of coronavirus patients were evident in each country, further contributing to the heterogeneity in study results. The disparate number of hospitals, available facilities, patient treatment strategies, and access to medications across different nations significantly influenced both patient numbers and mortality rates. Moreover, the accessibility of well-equipped medical centers varied among regions within a country, impacting the ability of high-risk groups, such as pregnant women, to receive timely and adequate care during the epidemic. The reluctance of many pregnant women to seek hospital care for check-ups and screening ultrasounds due to fear and anxiety during the pandemic introduces an additional layer of complexity that may affect the study's findings.
The retrospective design, single-center setting, and short sampling period were among the limitations of the study. Moreover, not all patients had access to serological and RT-PCR data to confirm SARS-CoV-2 infection. Another limitation was the lack of cooperation from experts and staff in the Department of Coronavirus and Infectious Diseases, due to their heavy workload during the pandemic. Despite these limitations, the study's strengths include the availability of RT-PCR results for every case and a sufficient sample size drawn from various regions of the province.
5.1. Conclusions
Based on the results of the present study, the incidence of maternal mortality and preterm birth in pregnant individuals with COVID-19 showed an increase. Therefore, accurate execution of prenatal care, training in health protocols, and attentive monitoring of both mother and fetus during and after delivery are necessary to reduce maternal and neonatal complications. More comprehensive research involving multiple hospitals within the same region is needed to verify the data and prevent sampling bias.