1. Background
Pregnancy is a critical and delicate period in a woman's life, with significant implications for both maternal and fetal health (1-3). During pregnancy, considerable physiological changes occur, including transformations in skin structure (2, 4, 5). One prominent skin change is the development of striae gravidarum, commonly known as pregnancy striae or stretch marks. Stretch marks are considered the most prevalent connective tissue change during pregnancy, with an estimated 50 - 90% of pregnant women experiencing them (6-9).
Stretch marks typically emerge in the late second trimester or early third trimester. However, some studies report that 43% of women may develop stretch marks before reaching 24 weeks of pregnancy (10-12). Histologically, stretch marks manifest in two stages. Initially, they appear as red or purple streaks, termed striae rubra. Over time, these marks fade into shiny, silver lines and form folds on the skin's surface, known as striae alba or permanent striae (2, 13). These marks can extend several centimeters in length and range from 1 to 10 mm in width (11, 14, 15).
Stretch marks may cause symptoms like itching, burning, and discomfort. They are commonly found on the abdomen, thighs, and breasts in women and may appear on the upper arms in men (16, 17).
Stretch marks, or striae, can occur under various physiological and pathological conditions (16, 18, 19). Although the exact cause of pregnancy striae is not fully understood, several factors, including genetics, hormonal changes, and physical stress on the skin, are believed to play key roles (2, 20, 21). Studies suggest that pregnancy-related hormones—such as estrogen, relaxin, and adrenal gland hormones—may contribute to striae formation in mechanically stressed areas by loosening collagen fiber connections and increasing the underlying substance (9, 18, 19).
Stretch marks often develop in areas where the skin is stretched due to factors such as sudden weight gain, certain endocrine disorders, or prolonged steroid use (4, 9, 22). Various other factors have been linked to stretch marks in pregnancy, including maternal age, infant birth weight, maternal race, skin type, gestational diabetes, poor nutrition, family history of striae, and delivery method; however, the impact of some of these factors remains inconclusive (2, 11, 23, 24).
While these skin changes are not medically harmful, they can significantly impact a woman's mental and emotional well-being (23, 25). Many women experience self-image issues, reduced self-confidence, and even depressive feelings due to these changes (17, 26). Stretch marks may affect perceptions of attractiveness, sexual response, and relationships with partners (9, 26, 27). Furthermore, such skin changes can lead to additional conditions during pregnancy, including the formation of papules and itchy urticarial plaques (27-29).
While some view these symptoms as a natural aspect of pregnancy, interventions for stretch marks mainly focus on prevention and treatment to minimize their appearance and severity during pregnancy (21, 28, 30). Today, various approaches are available for managing stretch marks, including exercise, proper nutrition, hydration, and a broad array of cosmetic and health products. However, some treatments, like microdermabrasion and microneedling, may not be accessible or affordable for everyone (28, 31, 32).
Some studies suggest a potential link between specific vitamins, particularly vitamin D, and the formation of striae (25, 31, 33, 34). Vitamin D, a fat-soluble vitamin primarily available in the forms of vitamin D₃ and vitamin D₂, belongs to the tocopherol family (34-36). Sunlight—specifically ultraviolet B rays—is the primary source of vitamin D, stimulating cholecalciferol production in the deeper epidermal layers (2, 36, 37). The biologically active form, 1,25-dihydroxyvitamin D, is essential for calcium homeostasis and bone health, and also serves as a stable marker for evaluating vitamin D levels in the body (33, 38, 39).
Additionally, vitamin D contributes to maintaining the epidermal permeability barrier, supporting keratinocyte proliferation and differentiation in the epidermis, as well as fibroblast proliferation in the dermis. Fibroblasts are key cells in the dermis responsible for collagen production and forming the extracellular matrix, both crucial for skin structure and resilience (20, 33, 40). Deficiencies in vitamin D and collagen are directly associated with the development of stretch marks, highlighting the role of vitamin D in skin integrity and stretch mark prevention (40, 41).
An article from Turkey suggests that maintaining adequate vitamin D levels may positively influence the reduction of striae in pregnant women (20). Given the factors discussed, the mixed results on the effectiveness of interventions (42-44), the limited research on vitamin consumption, and the high prevalence and cost of treating this condition with cosmetic products and medical treatments, a study to evaluate the effect of vitamin D on the severity of pregnancy striae is essential.
2. Objectives
This study was designed to determine the effect of vitamin D on the severity of pregnancy striae in the thigh region of primigravida women.
3. Methods
This research was a triple-blind randomized clinical trial conducted at Shahid Akbarabadi Hospital, a referral center for maternal health in Tehran, Iran, from July 20 to January 20, 2022. The study population included first-time pregnant women at 18 - 20 weeks of pregnancy. The study was registered in the Clinical Trial Center of Iran with the code IRCT20220509054799N1. Participants were first-time pregnant women aged 20 - 40, at 18 - 20 weeks of pregnancy, with a Body Mass Index (BMI) between 18.5 and 25, and possessing at least basic literacy skills.
Exclusion criteria included having pre-existing stretch marks, known systemic or underlying diseases (e.g., gestational diabetes, preeclampsia, adrenal gland disorder, Cushing’s disease), multiple pregnancies, history of skin conditions prior to stretch marks, known allergies to vitamin D, polyhydramnios, diagnosis of fetal macrosomia, use of other treatments or drugs for striae during the study, unwillingness to continue the intervention, adverse reactions to the cream (such as itching, burning, or redness), incorrect usage of the cream (not following the prescribed amount, frequency, or application method), and concurrent use of medications that interact with vitamin D (e.g., corticosteroids or anticoagulants).
Based on the Fitzpatrick Scale, which classifies skin types into six categories (31), we selected three general skin type categories: White, wheatish, and brown, with classification confirmed by a dermatologist. This study was conducted in a triple-blind manner, meaning that the researcher, the statistical consultant, and the participants were all unaware of the cream contents.
3.1. Sample Size
Based on previous studies and statistical requirements, the minimum sample size was calculated to achieve a 95% confidence level, 80% power, and an expected intervention effect of 6 units in the intervention group compared to the control, for statistical significance. Using the following formula (45), the sample size was determined to be 24 participants per group. To allow for a potential 20% sample loss, this was adjusted to 30 participants per group. Sampling was conducted continuously, with block randomization (blocks of 4 and 8) and allocation concealment managed through Sealed Envelope, ensuring that participants were randomly assigned to the intervention and placebo groups.
z0.975 = 1.96, z0.8 = 0.84, d = 6, s1 = s2 = 7.41
3.2. Intervention
Primiparous mothers entered the study between 18 - 20 weeks of pregnancy and were followed up over a 4-month period, with assessments at 4-, 8-, 12-, and 16-weeks post-intervention initiation. At the beginning of the study, participants completed a Personal Characteristics Questionnaire, and eligible women were randomly assigned to either the vitamin D cream group or the placebo group. The creams were prepared by a consultant pharmacist at the Faculty of Traditional Medicine, Iran University of Medical Sciences, following the researcher’s request. Both creams were identical in packaging, appearance, color, and smell to maintain blinding. Allocation was concealed using a site-generated code, with creams labeled as Group A and Group B. The vitamin D cream contained 5000 IU of topical vitamin D (approved by the Food and Drug Organization, registration IRC: 7976991642461548) mixed into 100 grams of Orand base cream (containing alcoacetyl, glycerin, triethanolamine, monostarch acid, white vaseline, and distilled water). The placebo cream used the same Orand base cream without vitamin D.
Participants were thoroughly informed about the study protocol and were advised that they might be randomly assigned to either intervention or placebo groups. The purpose of the study was clearly explained, and informed consent was obtained from all participants.
Participants were instructed to apply the creams twice daily, at 12-hour intervals, for the 4-month duration. They were trained to apply a thin layer of cream to the thigh area with their fingers, without massaging, and to avoid washing the area until the cream was absorbed. The investigator provided in-person guidance on accurately completing daily checklists during the initial visit.
Both the researcher and the participants were blinded to the cream contents. The severity of striae erythema was assessed by the researcher using Atwal's Numerical Tool Scale, and participants recorded each cream application in a daily checklist provided to them.
The Atwal tool was used at 4-, 8-, 12-, and 16-weeks post-intervention to measure the severity of striae erythema on the thighs. Participants visited the perinatology clinic at Shahid Akbarabadi Center for these assessments, where the researcher reviewed each participant’s checklist to monitor cream usage. Participants were expected to use one can of cream per month on average. Visits were aligned with regular pregnancy checkups, and weight was measured at each stage due to its potential effect on striae development.
The researcher maintained weekly contact with participants via calls or messages to confirm adherence to cream application, ensure checklist completion, and inquire about any possible side effects, such as itching or redness. Participants were instructed to immediately report any adverse effects by phone. Sampling continued until the final sample size was reached, accounting for any potential dropouts in both groups. At the conclusion of the study, BMI, gestational age, and a final observation record of pregnancy striae severity were completed by the researcher.
3.3. Outcome Measurement
Data collection instruments included the Atwal Striae Gravidarum Score, a Demographic Questionnaire, and a consumer checklist. The researcher, who was thoroughly trained, assessed and documented the severity of striae erythema at each stage using Atwal’s numerical tool. The maximum score for each area was 3, with scoring as follows: No erythema (score 0), moderate erythema (light red or pink, score 1), symptomatic erythema (dark red, score 2), and severe erythema (purple, score 3) (19).
To ensure validity, the face and content validity of the Personal Characteristics Questionnaire were evaluated. The questionnaire was reviewed by seven faculty members from Iran University of Medical Sciences. Following their feedback and final assessments, necessary modifications were made to the Demographic Questionnaire and daily checklist. The accuracy of the Atwal Striae Gravidarum Score tool, initially validated by Atwal et al., was confirmed to be 78.2% (19).
To assess reliability, inter-rater reliability was used. The investigator and a specialist dermatologist independently evaluated and documented the presence, amount, and severity of pregnancy-induced stretch marks in 10 subjects. The findings demonstrated high agreement, resulting in an intraclass correlation coefficient (ICC) of 0.98 - 1.0 for all regions.
The Demographic Questionnaire gathered information on participants' age, economic status, education, number of pregnancies, history of abortion, presence of stretch marks in first-degree relatives, and skin tone. It also included gestational age at examination times, mother’s weight before and after the intervention, height (for BMI calculation), type of intervention (A or B), and duration of cream usage. This data was collected by the researcher through interviews with participants. Additionally, a consumer checklist was provided for participants to record the daily frequency of cream application, facilitating accurate tracking of adherence to the intervention protocol.
3.4. Statistical Analysis
Data analysis was conducted using SPSS version 16 software. Descriptive statistics, including absolute and relative frequency, mean, standard deviation, minimum, maximum, and mean rank, were applied. For analytical statistics, the chi-square test, Mann-Whitney U test, Fisher’s exact test, and independent t-test were utilized.
The Kolmogorov-Smirnov test indicated a non-normal data distribution, so the non-parametric Mann-Whitney U test was used to compare the incidence of striae between the two groups. The chi-square test compared education and midwifery characteristics, Fisher’s exact test assessed economic status, and the independent t-test was used to compare age and BMI between the groups. The level of significance was set at P < 0.05.
This study adhered to the principles of the Declaration of Helsinki. Confidentiality was strictly maintained, and informed, written consent was obtained from all participants, who were assured of their freedom to withdraw from the study at any time without any impact on the care provided. Ethics approval was granted by the Regional Research Ethics Committee at Iran University of Medical Sciences (IUMS) under the code IR.IUMS.REC.1401.215.
4. Results
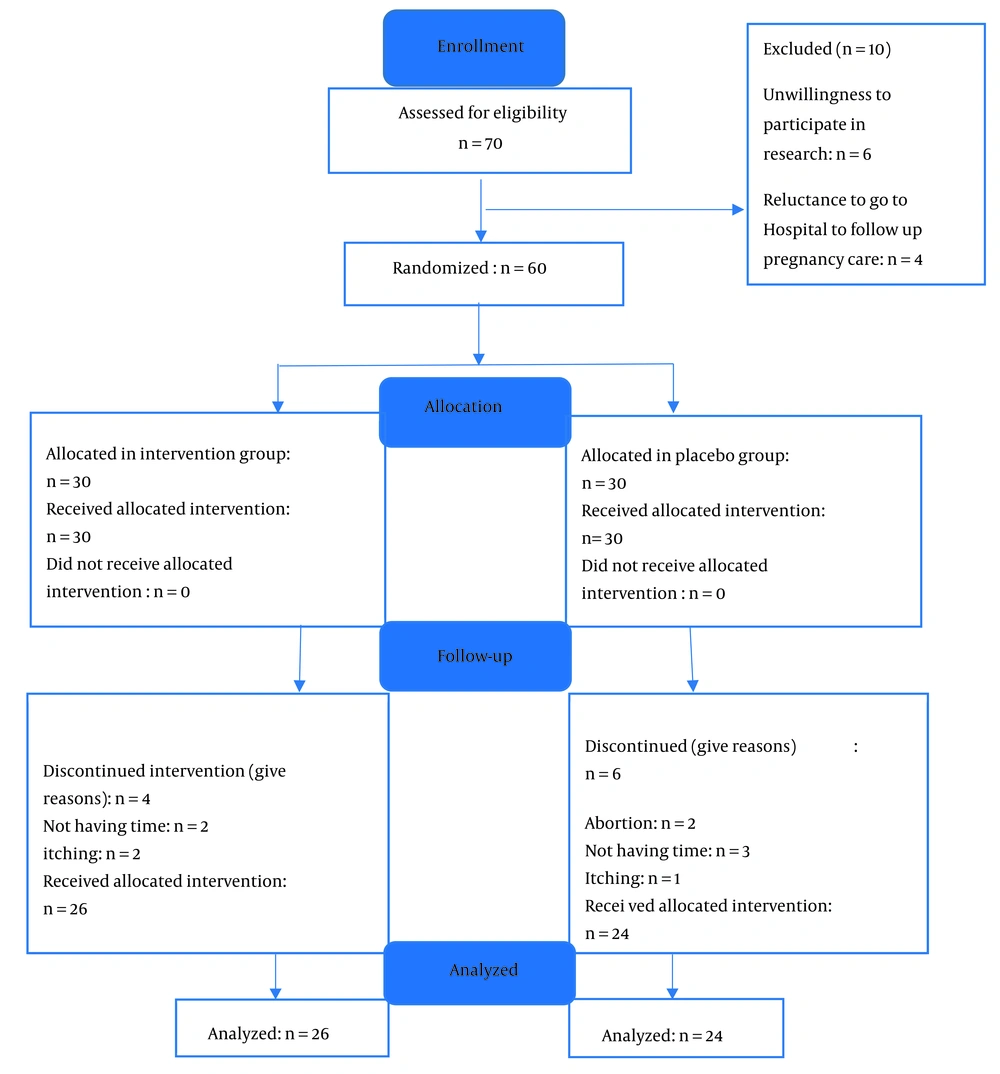
In this study, 70 patients were initially enrolled. After assessing eligibility, 10 patients were excluded, leaving 60 participants—30 in the intervention group and 30 in the control group. However, the sample size decreased further, resulting in a final analysis of 50 patients evenly distributed between the two groups (Figure 1).
Table 1 presents the demographic and obstetric characteristics of participants in both groups. Since these characteristics were normally distributed according to the Kolmogorov-Smirnov test, parametric tests were used to compare the two groups. The results showed that the majority of participants in both groups were aged 20 to 25, held a diploma, and had a relatively sufficient income. After the intervention, most participants in both groups were classified as overweight, with a BMI between 25 and 29.9 kg/m². A greater proportion of participants in the vitamin D group had a history of miscarriage, which was less common in the placebo group. Additionally, most participants in both groups reported a family history of striae in first-degree relatives and had white skin.
| Groups | Demographic and Obstetrics Characteristics | The Significance Level | |
|---|---|---|---|
| Placebo Number | Vitamin D Number | ||
| Age (y) | |||
| Mean ± Standard deviation, minimum - maximum | 28.65 ± 4.82, 22 - 40 | 27 ± 5.18, 20-37 | Independent t-test, P = 0.228, df = 0.481, t = -1.233 |
| Education | |||
| Elementary | 6 (25) | 8 (30.8) | Chi-square test, P = 0.945, df = 3, X2 = 0.376 |
| Middle | 5 (20.83) | 4 (15.4) | |
| Diploma | 8 (33.33) | 9 (34.6) | |
| University | 5 (20.83) | 5 (19.2) | |
| Income | |||
| Enough | 1 (4.17) | 1 (8.3) | Fisher's test, P = 0.722 |
| Insufficient | 5 (20.83) | 3 (11.5) | |
| Relatively enough | 18 (75) | 22 (84.6) | |
| Body Mass Index after the intervention (kg/m2) | |||
| Mean ± standard deviation minimum - maximum | 27.48 ± 1.57, 23.91 - 29.48 | 26.61 ± 2.05, 22.37 - 29.55 | Independent t-test, P = 0.101, df = 48, t = -1.674 |
| History of abortion | |||
| Yes | 11 (45.83) | 15 (57.7) | Chi-square test, P = 0.403, df = 1, X2 = 0.703 |
| No | 13 (57.17) | 11 (42.3) | |
| History of stretch marks | |||
| Has it | 19 (73.08) | 14 (53.8) | Chi-square test, P = 0.059, df = 1, X2 = 4.98 |
| Has no | 5 (16.67) | 12 (46.2) | |
| Skin types | |||
| Brown | 6 (25) | 8 (30.8) | Chi-square test, P = 0.360, df = 2, X2 = 7.41 |
| White | 10 (41.67) | 14 (53.8) | |
| Wheatish | 8 (33.33) | 4 (15.4) | |
Statistical analysis indicated no significant differences between the two groups in terms of demographic and obstetric characteristics, including age, education level, economic status, BMI after the intervention, history of abortion, family history of stretch marks, and skin tone.
The severity of pregnancy striae erythema was compared between the two groups, as shown in Table 2. Given that the severity scores were not normally distributed according to the Kolmogorov-Smirnov test, the non-parametric Mann-Whitney test was used for analysis. Results indicated no statistically significant differences in the severity of pregnancy stretch marks between the two groups at 4, 8, 12, and 16 weeks after the intervention began, as confirmed by the Mann-Whitney test (Table 2).
| Groups | Atwal Scale | The Result of the Mann-Whitney Test | |
|---|---|---|---|
| Placebo | Vitamin D | ||
| Four weeks after the start of the intervention | |||
| No erythema (score 0) | 20 (83.33) | 25 (96.2) | |
| Moderate erythema (score 1) | 4 (16.67) | 1 (3.8) | |
| Mean Rank, 25th-75th | 27.17, 0.000 - 0.000 | 23.96, 0.000 - 0.000 | P = 0.1, Z = -1.495 |
| Eight weeks after the start of the intervention | |||
| No erythema (score 0) | 20 (83.33) | 24 (84.62) | |
| Moderate erythema (score 1) | 4 (16.67) | 2 (15.38) | |
| Mean Rank, 25th -75th | 26.67, 0.000 - 0.000 | 24.42, 0.000 - 0.000 | P = 0.3, Z = -0.966 |
| Twelve weeks after the start of the intervention | |||
| No erythema (score 0) | 20 (83.33) | 24 (84.62) | |
| Moderate erythema (score 1) | 4 (16.67) | 2 (15.38) | |
| Mean Rank, 25th-75th | 26.67, 0.000 - 0.000 | 24.42, 0.000 - 0.000 | P = 0.3, Z = -0.966 |
| Sixteen weeks after the start of the intervention | |||
| No erythema (score 0) | 16 (66.67) | 23 (76.92) | |
| Moderate erythema (score 1) | 8 (33.33) | 3 (23.08) | |
| Mean Rank, 25th-75th | 28.33, 0.000 - 0.000 | 22.88, 0.000 - 0.000 | P = 0.06, Z = -1.840 |
a Values are expressed as No. (%).
5. Discussion
The objective of this study was to assess the effect of vitamin D cream on the severity of pregnancy stretch marks on the thighs of first-time pregnant women. Results showed no significant difference in the severity of pregnancy stretch marks between the vitamin D cream group and the placebo group, leading to the conclusion that vitamin D does not have a reducing effect on the severity of pregnancy stretch marks.
Although research in this area is limited, a related study conducted in Arak, Iran, examined the Effect of Sesame and Sweet almond Oil on the Prevention of Stretch Marks and Itching in First-Time Mothers. In that study, which included women at 16 - 20 weeks of pregnancy, the use of sesame oil alone or combined with sweet almond oil was shown to significantly reduce the occurrence of stretch marks and itching, as compared to a placebo (42). The results suggest that these oils were effective in reducing abdominal stretch marks and associated discomfort.
These findings align with the potential impact of topical compounds on the severity of pregnancy striae, possibly through effects on collagen production—a process influenced by vitamin D. While sesame and almond oils contain vitamin D, it is important to note that this study did not isolate vitamin D as the sole component, and the area of application differed.
A study conducted in Mashhad, Iran, aimed to investigate the effect of a topical herbal cream (comprising calendula, chamomile, geranium rose, cocoa butter, and soybeans) on the incidence and severity of pregnancy striae in primiparous women during the first trimester. The findings indicated a significant difference in striae severity between the group using the combined herbal cream and the placebo group using a standard moisturizing cream (43). These results do not align with the findings of the present study regarding the use of topical compounds for managing the severity of pregnancy striae, despite the presence of vitamin D in chamomile.
However, this inconsistency may not be conclusive, as the study in Mashhad did not isolate vitamin D as the sole active ingredient, and the area of application differed from that of the present study.
A study conducted in Tehran, Iran, evaluated the effectiveness of olive oil and teak cream on pregnancy striae on the abdominal skin in nulliparous women during the second trimester (18 - 20 weeks of pregnancy). By the end of the second trimester, striae developed in 40% of the olive oil users, 16.7% of the teak cream users, and 56% of the control group. The study found no significant difference in the incidence of pregnancy striae between the two intervention groups and the control group (44). These results are consistent with the findings of the present study regarding the use of topical compounds for managing the incidence and severity of pregnancy striae, despite the presence of vitamin D in olive oil.
A study conducted in Shahrekord, Iran, found that aloe vera and sweet almond oil creams were more effective in reducing itching and erythema and preventing stretch marks on the abdominal area compared to a base cream and a control group. However, all three creams had similar effects on the diameter and number of striae. Notably, aloe vera and sweet almond oil creams showed a greater effect in reducing striae erythema than the base cream (46). These results do not align with the findings of the present study regarding the use of topical compounds for managing the severity of pregnancy striae. Although almond oil is rich in vitamin D, it was not used as an isolated component, and the area studied differed from the current research. Therefore, the inconsistency between these results cannot be fully interpreted as contradictory. Additionally, erythema in the Shahrekord study was assessed qualitatively through observation rather than a specific tool, potentially introducing bias.
The results of this study did not support the effectiveness of vitamin D in reducing the severity of pregnancy striae on the thigh area. Consequently, adding vitamin D to a base cream may not enhance its preventative effects against pregnancy-related striae. Strengths of this study include the use of a placebo, randomized assignment of participants to groups, as well as blinding and concealment in sample handling.
The limitations of this study include several factors. First, participant concerns were notable, as some participants expressed hesitancy about revealing personal information, which may have influenced their responses. Additionally, comprehension issues arose, as individuals with lower education levels may have struggled to understand certain questions, potentially affecting the accuracy of their responses. Another limitation was the lack of control over serum vitamin D levels, which were not consistently monitored and could have impacted the results. Furthermore, inconsistent cream application was observed, as the frequency of cream application was not accurately tracked, affecting adherence. Lastly, the absence of a control group with no intervention limited the ability to definitively assess the effectiveness of vitamin D in comparison to no treatment.
This research suggests exploring the effects of vitamin D creams on other body areas or over extended periods, as well as investigating their use in treating postpartum stretch marks. It may also be valuable to study the effectiveness of these creams among different populations, such as women aiming to alter their weight or athletes concerned about stretch marks. Additionally, further studies could examine the impact of these creams on women with multiple pregnancies.
The findings of this study are relevant to pregnant women, gynecologists, midwives, healthcare providers, and those responsible for educational and support programs for pregnant women. Helping pregnant women is a core objective in midwifery and healthcare, and preventing and treating pregnancy-related stretch marks is an essential aspect of this support. Given the high prevalence of stretch marks during pregnancy and the significant costs associated with cosmetic products and medical treatments, using vitamin D supplements may offer a practical and accessible option for prevention.