1. Background
Adolescence is a critical developmental stage characterized by significant physical and emotional changes for teenagers (1). During this period, the presence of chronic disorders like polycystic ovary syndrome (PCOS) can greatly impact adolescents' self-esteem and overall quality of life (2). The PCOS is the most common endocrine disorder affecting reproductive-aged women, with reported prevalence rates of approximately 3.4% to 19.6% among young girls (3). Various theories have emerged to explain the etiology of this syndrome, including factors such as insulin resistance, genetic predisposition, environmental influences, nutrition, and physical activity (4). However, the importance of genetics, nutrition, and physical activity cannot be overstated. Irregular menstrual cycles and hyperandrogenism serve as the gold standard for diagnosing PCOS (5, 6). Given the serious health implications of PCOS—ranging from obesity and metabolic syndrome to diabetes, infertility, and mental health issues—adopting a risk factor-based management approach during adolescence is essential to prevent these outcomes (7). Promoting reproductive health and overall well-being among adolescents is crucial for ensuring their quality of life and preventing future health complications (8).
Despite the clear necessity for intervention, there is limited data on the nutritional and dietary patterns of adolescents affected by PCOS (9). Existing research primarily focuses on adult women with PCOS, indicating that they experience higher energy intake and a more sedentary lifestyle compared to their unaffected peers (10). Studies conducted in India suggest that young women with PCOS tend to be physically inactive, often classified as obese, with their average nutrient intake falling below recommended levels (11, 12). Additionally, an Iranian study demonstrated a significant association between suboptimal dietary habits and an increased risk of developing PCOS (13). This investigation revealed that women with PCOS had higher intakes of unhealthy fats (including saturated and monounsaturated fats) and refined carbohydrates, while simultaneously consuming lower amounts of essential vitamins and minerals such as vitamins A, B5, B6, B12, C, D, as well as potassium, protein, cholesterol, and fiber. These dietary imbalances likely contribute to the metabolic and reproductive health issues frequently observed in women with PCOS (14).
Recognizing the importance of micronutrients and macronutrients, it is vital to assess the dietary composition of teenage girls with PCOS to tailor nutrition plans that effectively address their unique needs. There is a clear research gap, as there is a dearth of studies focusing on the dietary habits and lifestyles of adolescents affected by PCOS. This study aims to compare the physical activity levels and dietary patterns of adolescents diagnosed with PCOS to those without the condition. By doing so, we seek to gain a deeper understanding of how nutrition influences the management of PCOS within this specific population.
2. Objectives
This study aimed to compare nutritional patterns, including macronutrient intake and intensity of physical activity, among adolescents with PCOS and those unaffected by the condition.
3. Methods
The study was conducted in full compliance with the ethics code (IR.TUMS.FNM.REC.1400.190) obtained from the Ethics Committee of the Faculty of Nursing and Midwifery and Rehabilitation School of Tehran University of Medical Sciences, Iran. Approval was also secured from the Health Department at Golestan University of Medical Sciences. This cross-sectional study took place in Golestan Province, Iran, involving 238 adolescents aged 12 - 24 years. Data collection began in March 2022 and was completed by December 2022, allowing us to gather information across various seasonal variations that might influence physical activity levels and dietary habits.
Inclusion criteria required adolescents aged 12 to 24 years with no history of known diseases affecting the menstrual cycle, such as endocrine disorders or genetic problems. The diagnosis of PCOS was based on the Rotterdam criteria, which require the presence of at least two of the following three features: Oligo/anovulation, hyperandrogenism, and polycystic ovarian morphology (15). Participants who did not meet these criteria or had any of the following exclusion criteria—such as having other endocrine disorders, known metabolic conditions, or confounding health issues—were not included in the study.
The total sample size for this study was calculated to be 238 participants, including 119 participants in each group. We used the study outlined by Maya et al. (14). The calculation was based on the following parameters: A significance level (α) of 0.05, which corresponds to a Z-value of 1.96 for a two-tailed test, a power (1 - β) of 0.84, and P1 (25%), which refers to the prevalence of low physical activity in adolescents with PCOS, while P2 (10%) indicates the prevalence of low physical activity in adolescents without PCOS. Using these values, the sample size was determined with a 20% dropout rate to ensure adequate statistical power to detect meaningful differences between the groups.
For data collection, we utilized a demographic questionnaire, the Adami and Cordera Nutrition Questionnaire, and the Azad-Fesharaki Physical Activity Questionnaire (AFPAQ). The demographic questionnaire captured essential information, including age, weight, height, Body Mass Index (BMI), education level, family history of PCOS, and menstrual status (duration of bleeding and intervals between cycles). The Adami and Cordera Nutrition Questionnaire assessed dietary intake, food patterns, and eating habits (16). The Persian version of the Adami and Cordera Nutrition Questionnaire has undergone rigorous testing for both validity and reliability (17). The accuracy of translation was confirmed using the method of translation and retranslation. The questionnaire demonstrated strong construct validity through factor analysis, with a KMO value of 0.931, exceeding the cutoff point of 0.9, indicating that the data were suitable for factor analysis. Additionally, the Bartlett test result was significant (P < 0.001). In this regard, principal component analysis via Varimax rotation indicated that all factors are compatible with the desired factor (8), accurately measuring dietary intake as intended. Regarding reliability, a Cronbach's alpha coefficient of 0.86 was reported, indicating excellent internal consistency among the items in the questionnaire. The questionnaire typically contains approximately 40 questions that cover multiple dimensions of nutrition and eating patterns (18). The questionnaire typically contains approximately 40 questions that cover multiple dimensions of nutrition and eating patterns. Responses to these questions are reported in terms of frequency, quantity, and types of food consumed, allowing for a comprehensive assessment of dietary habits. By analyzing the answers, we can interpret the nutritional status of participants, identifying areas such as nutrient intake, dietary variety, and adherence to dietary guidelines. This analysis helps to reveal potential deficiencies or excesses in nutrition, providing valuable insights into the overall health and eating behaviors of the individuals surveyed.
The AFPAQ, developed by Gholami Fesharaki and Azad Marzabadi, was employed to obtain information regarding physical activity levels, categorized as low, moderate, and high. This questionnaire contains 13 questions. The method for using the questionnaire is as follows: To score and utilize the questionnaire effectively, first, the questions (except for question 1) are rated from 1 to 5. Then, by summing questions 1 to 4, the index for physical activity during work is calculated; by summing questions 5 to 8, the index for fatigue is created; and by summing questions 9 to 13, the index for leisure-time physical activity is established. (question 1 is scored from 5 to 1).
Characteristics of groups with low, moderate, and high physical activity levels: (1) Low: Individuals in this group have a total score of 0 - 10, indicating they may spend most of their day engaged in sedentary activities with little to no regular exercise; (2) moderate: Individuals in this category have a total score of 11 - 20, suggesting they participate in activities such as brisk walking, recreational sports, or structured exercise sessions several times a week; (3) high: Individuals in this group score 21 or higher, indicating they likely engage in structured exercise programs, competitive sports, or physically demanding jobs.
The CVR coefficient for this questionnaire was 60%. Explanatory factor analysis revealed three factors—physical activity at work, physical activity at leisure time, and exhaustion—with a total variance of 45% and a Kaiser-Meyer-Olkin Index of 71%. These factors were confirmed by confirmatory factor analysis (AGFI = 0.963, RMSEA = 0.053). The reliability of the questionnaire was 70%, as assessed by Cronbach's alpha, while the correlation coefficient for the test-retest method was 87% (19).
To ensure comprehensive data collection, researchers made reasonable efforts to follow up with participants, encouraging completion of the questionnaire. Documentation of data collection attempts and protocols for addressing missing data were rigorously followed. Data collection was conducted in nine randomly selected health centers in Gorgan, chosen from twenty-two available centers using a drawing method. Trained research personnel approached families, reviewed their electronic files, and sought parents' willingness for their children to participate, ensuring that their information would remain confidential. Written informed consent was obtained from all participants, who were then randomly assigned into two groups using a convenient method.
To diagnose PCOS, the applicable Rotterdam criteria were utilized, with diagnosis confirmed through sonographic and laboratory tests evaluated by qualified gynecologists. Participants diagnosed with PCOS were categorized into the affected group, while those not diagnosed were placed in the non-affected group. Adolescents in both groups completed the three demographic and assessment questionnaires through self-reporting.
The collected data were meticulously entered into SPSS version 22 software for comprehensive analyses and comparisons between the two groups. Descriptive statistical methods, including frequency distribution tables, means, and standard deviations, were employed. Additionally, the independent t-test was used for evaluating differences in quantitative variables between groups that followed a normal distribution, while the chi-square test was applied for assessing relationships between qualitative variables. The Mann-Whitney test was applied for variables with a non-normal distribution. The chi-square test was employed to compare qualitative variables between the groups. For assessing normality of variables with low frequency, the Shapiro-Wilk test was utilized. The threshold for statistical significance was set at P < 0.05.
4. Results
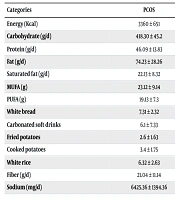
In the current study, 238 teenagers participated, with a mean age of 19.16 ± 3.18 years. Their demographic characteristics are outlined in Table 1. The results indicate that the PCOS group significantly differed from the non-PCOS group in terms of weight, height, BMI, and menstrual characteristics (P < 0.05). As shown in Table 2, adolescents with PCOS had significantly higher intake of energy, carbohydrates, and fats, but lower intake of protein compared to those without PCOS (P < 0.05). Furthermore, the findings indicate that while there were no significant differences in sedentary and light activity levels between the two groups, participants with PCOS exhibited significantly lower levels of moderate, vigorous, and total daily physical activity (Table 3).
| Variables | Non-PCOS | PCOS | Statistically Tests |
|---|---|---|---|
| Age (y) | 19.02 ± 3.29 | 19.29 ± 3.10 | P = 0.611 b; t = 0.511 |
| Weight (kg) | 57.37 ± 5.20 | 80.49 ± 6.95 | P = 0.001 b; t = 23.05 |
| Height (m) | 158.41 ± 6.43 | 161.56 ± 6.26 | P = 0.003 b ; t = 3.04 |
| BMI (kg/m2) | 22.96 ± 2.68 | 30.94 ± 3.38 | P = 0.001 b; t = 22.603 |
| Menarch (y) | 12.49 ± 1.162 | 12.78 ± 1.150 | P = 0.09 b, t = 2.691 |
| Interval between two mensuration (d) | 27.04 ± 2.68 | 36.02 ± 2.44 | P = 0.001 b; t = -3.330 |
| Length of menstruation (d) | 5.94 ± 1.07 | 3.54 ± 0.68 | P = 0.001 b; t = 17.88 |
| Education | P = 0.821 c | ||
| Under diploma | 34 (17.3) | 40 (20) | |
| Diploma | 70 (34.7) | 64 (32) | |
| University | 96 (48) | 96 (48) | |
| Family history of PCOS | χ2 = 356.55; P = 0.337 d | ||
| Yes | 32 (16) | 44 (22) | |
| No | 168 (84) | 156 (78) |
Abbreviations: PCOS, polycystic ovary syndrome; BMI, Body Mass Index.
a Values are expressed as mean ± SD or No. (%).
b Independent t-test.
c Mann-Whitney.
d Chi-square.
| Categories | PCOS | No-PCOS | OR | 95% CI | P-Value b |
|---|---|---|---|---|---|
| Energy (kcal) | 3360 ± 651 | 2099 ± 409 | 2.31 | (2.11 - 2.98) | < 0.001 |
| Carbohydrate (g/d) | 418.30 ± 45.2 | 234.3 ± 61.08 | 6.38 | (4.02 - 10.25) | < 0.001 |
| Protein (g/d) | 46.09 ± 13.83 | 87.18 ± 23.13 | 0.25 | (0.17 - 0.37) | < 0.001 |
| Fat (g/d) | 74.23 ± 28.26 | 58.12 ± 17.02 | 1.64 | (1.01 - 2.68) | < 0.001 |
| Saturated fat (g/d) | 22.13 ± 8.32 | 14.13 ± 5.87 | 1.91 | (1.25 - 2.92) | < 0.001 |
| MUFA (g) | 23.12 ± 9.14 | 21.05 ± 1.21 | 1.12 | (0.78 - 1.63) | 0.34 |
| PUFA (g) | 19.13 ± 7.3 | 17.11 ± 1.482 | 1.15 | (0.89 - 1.49) | 0.14 |
| White bread | 7.31 ± 2.32 | 6.12 ± 1.63 | 1.27 | (0.74 - 2.18) | 0.50 |
| Carbonated soft drinks | 6.1 ± 7.33 | 4.29 ± 3.63 | 1.64 | (1.02 - 2.64) | 0.04 |
| Fried potatoes | 2.6 ± 1.63 | 0.73 ± 1.63 | 4.88 | (1.24 - 19.22) | 0.04 |
| Cooked potatoes | 3.4 ± 1.75 | 1.23 ± 1.13 | 3.44 | (1.01 - 11.53) | 0.04 |
| White rice | 6.32 ± 2.63 | 2.65 ± 1.63 | 2.82 | (1.10 - 7.18) | 0.03 |
| Fiber (g/d) | 21.04 ± 11.14 | 29.19 ± 17.7 | 0.60 | (0.37 - 1.01) | 0.17 |
| Sodium (mg/d) | 6425.36 ± 1394.36 | 4821.16 ± 1822.4 | 1.92 | (1.24 - 2.97) | < 0.001 |
Abbreviations: PCOS, polycystic ovary syndrome; OR, odds ratio; CI, confidence interval; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids.
a Values are expressed as mean ± SD.
bt-test.
| Physical Activity Levels | PCOS a | No-PCOS a | P-Value |
|---|---|---|---|
| Sedentary and light activity (h/wk) | 18 ± 3 (16 - 24) | 35 ± 4 (24 - 39) | 0.07 b |
| Moderate physical activity (min/wk) | 30 ± 4 (24 - 40) | 50 ± 6 (45 - 60) | < 0.001 c |
| Vigorous physical activity (min/wk) | 20 ± 3 (20 - 35) | 45 ± 4 (30 - 50) | < 0.001 c |
| Sitting and lying down (h/wk) | 45 ± 5 (40 - 50) | 35 ± 3 (30 - 50) | 0.64 c |
| Total daily physical activity (h/d) | 2.69 ± 0.56 (0.648) | 3.97 ± 0.85 (0.692) | < 0.001 c |
Abbreviations: PCOS, polycystic ovary syndrome; CI, confidence interval.
a Values are expressed as mean ± SD (95% CI).
b Mann‐Whitney U.
ct‐test.
5. Discussion
The study results suggest that adolescents with PCOS exhibit differences in anthropometric measures (height, weight, BMI) and menstrual cycles compared to their non-PCOS counterparts. However, while these discrepancies may indicate a relationship between PCOS and these factors, it is crucial to note that they do not necessarily imply causation. The observed differences in these factors between the PCOS and non-PCOS groups may be associated with the hormonal and metabolic disturbances typical of PCOS. This finding is significant as it sheds light on the distinct physical and reproductive differences manifesting in adolescents with the condition (20-25). For instance, an Iranian study reported that adolescent girls with PCOS had higher weight, height, BMI, and greater menstrual dysfunction (22). Similarly, a Thai study corroborated these findings (23). Despite the consistency of these results across diverse cultural and geographical contexts, two other studies reported no significant differences in weight and height between the groups (26, 27). This discrepancy could be due to various factors, including variations in sample sizes and potential racial/ethnic differences in the populations studied.
While the anthropometric findings were mixed, there was a consensus on the presence of menstrual dysfunction in adolescents with PCOS compared to their non-PCOS peers. As mentioned, the affected girls in our study reported high levels of carbohydrate and fat intake, alongside low protein consumption. An excessive intake of these nutrients can lead to weight gain, which may contribute to obesity, insulin resistance, and hormonal imbalances associated with PCOS (28). Notably, obesity—particularly visceral (abdominal) adiposity—is prevalent among women with PCOS, regardless of their overall body weight. This excess adiposity exacerbates the metabolic and reproductive challenges linked to PCOS. The presence of obesity increases insulin resistance and induces compensatory hyperinsulinemia (elevated insulin levels), which promotes further fat accumulation and hampers fat breakdown (29).
Additionally, other research indicated that a high intake of calories from fat, particularly saturated fat and cholesterol, can heighten the risk of developing PCOS (30). However, in one study, researchers reported that total calorie intake in women with PCOS did not significantly differ from that of healthy women when adjusted for body weight. The key distinction appears to lie in the consumption of junk food and fiber-poor foods among women with PCOS (31). This suggests that calorie consumption alone may not account for the disparities in metabolic outcomes observed in these populations. A key distinction appears to lie in the dietary choices, particularly the higher consumption of junk food and fiber-poor foods among women with PCOS. This difference in dietary patterns could contribute to variations in insulin sensitivity and overall metabolic health, indicating that the quality of calories consumed may play a crucial role in explaining the differences in findings between the two groups.
The study also identified differences in physical activity levels between the two groups. This finding is consistent with several other studies that have examined physical activity in adolescents. For example, research conducted in 2019 (32) and 2021 (33) found that adolescents with PCOS often have lower physical activity levels and higher rates of sedentary behavior than their healthy counterparts. These variations highlight the importance of addressing lifestyle factors, as they can significantly impact the health and well-being of adolescents affected by PCOS. Contrasting the findings of the present study, a previous investigation in 2021 found no relationship between physical activity and PCOS. The differences in outcomes may relate to factors such as sample size, measurement tools used, and the thresholds for physical activity required to influence PCOS. A review showed that physical inactivity and poor dietary habits significantly impact PCOS development (34).
In our findings, while levels of inactivity and low physical activity were similar between the affected and healthy groups, moderate to high levels of physical activity were significantly lower in the PCOS group. These results resonate with a study from 2022, which found a robust connection between a sedentary lifestyle and the severity of PCOS symptoms (35). Regular exercise offers numerous benefits for individuals with PCOS, including increased menstrual frequency and/or ovulation, improved insulin sensitivity, reduced hyperinsulinemia, and enhanced mental well-being (36-38). Exercise also helps regulate key sex hormones (FSH and free testosterone) and improve other PCOS-related complications (37). The beneficial mechanisms of exercise include reducing body fat and increasing the secretion of beta-endorphins, which lower LH and testosterone levels (32, 38).
The limitations of the current study are multifaceted. One significant limitation is the reliance on self-reported data, which can introduce biases such as social desirability bias or inaccuracies in reporting dietary habits and physical activity levels. This reliance may affect the validity of the findings, as participants might underreport unhealthy behaviors or overreport healthy ones. Additionally, the cross-sectional design of the study limits the ability to draw causal inferences about the relationships between diet, physical activity, and PCOS. Without longitudinal data, it is challenging to determine whether the observed associations reflect direct causative effects or are influenced by other confounding variables. Moreover, potential non-cooperation from students and parents, along with strict school policies, may have restricted researchers' access to the study environment, potentially leading to a selection bias. This limitation could affect the generalizability of the findings to a broader adolescent population.
Despite these limitations, this study represents an effort to conduct research on teenagers with PCOS, raising awareness about this condition. A more comprehensive understanding of these limitations will contribute to a balanced discussion of the study's findings and their implications for future research.
5.1. Conclusions
The results of the current study indicate significant differences in physical activity status, nutritional patterns, and total calorie intake between adolescents with PCOS and those without the condition. Specifically, adolescents with PCOS demonstrated lower levels of moderate to high physical activity compared to their non-PCOS counterparts, which may contribute to the metabolic and reproductive challenges associated with the syndrome. Additionally, the study found that affected adolescents reported higher carbohydrate and fat intake, coupled with lower protein consumption, which could exacerbate issues such as obesity and insulin resistance commonly seen in PCOS. These findings underscore the importance of addressing lifestyle factors, including diet and physical activity, in the management of PCOS among adolescents.
The observed discrepancies in nutritional patterns and physical activity levels not only highlight the unique challenges faced by this population but also suggest potential avenues for targeted interventions aimed at improving health outcomes. In conclusion, this study serves as a foundational effort to explore the interplay between diet, physical activity, and PCOS in adolescents, raising awareness of the condition and emphasizing the need for further research. Future studies should aim to delve deeper into these associations, exploring the impact of specific dietary interventions and structured physical activity programs on the management of PCOS symptoms in this demographic. By doing so, we can better inform clinical practices and develop effective strategies to support adolescents affected by PCOS.
5.2. Future Research Directions
- Longitudinal studies: Conduct longitudinal studies to establish causal relationships between dietary habits, physical activity, and the development or progression of PCOS in adolescents.
- Dietary interventions: Investigate the specific effects of various dietary patterns and interventions on PCOS symptoms and management. This could involve controlled trials focusing on low-carb or high-fiber diets.
- Physical activity guidelines: Explore the impact of different types and intensities of physical activity, particularly structured exercise programs, on hormonal regulation and symptom management in adolescents with PCOS.
- Diverse populations: Expand research to include diverse racial and ethnic groups to better understand how these factors interact with PCOS prevalence and management.
By addressing these areas, future studies can provide deeper insights into the multifaceted nature of PCOS and inform more effective prevention and intervention strategies.