1. Background
Diabetes is the most common chronic and noncommunicable disease globally and has become a significant public health problem in recent centuries (1). The global incidence of diabetes was estimated at 536.6 million people in 2021 and is projected to increase to 783.2 million by 2045 (2). In Iran, a study found a diabetes prevalence ranging from 9.9% to 14.4%, with this number expected to double by 2045 (3). The prevalence of diabetes increases with age, peaking in older adults (4), where nearly a quarter of those over 65 years of age are affected by the disease (5, 6).
Diabetic nephropathy, neuropathy, retinopathy, and cardiomyopathy are among the serious complications that often arise in individuals with diabetes (7). Clinical data also suggest that 30% to 40% of patients will experience at least one complication approximately 10 years after the onset of diabetes (8). It is crucial to provide self-care support to individuals with type 2 diabetes to enable them to take an active role in managing their health (9).
Diabetes self-care education (DSCE) is one of the most effective strategies for managing diabetes-related complications and improving glycemic control (10). It encompasses a range of complex behaviors, including dietary changes, physical activity, medication adherence, blood glucose monitoring, and foot care (11, 12). Numerous studies have found that adults with type 2 diabetes often have inadequate DSCE, resulting in poor glycemic control (13). Several factors contribute to suboptimal self-care among diabetics, such as low motivation, insufficient resources for decision-making, inadequate knowledge about diabetes, poor communication between patients and physicians, and competing daily tasks (14).
Smartphones and related technologies are being utilized to address these issues. Digital health interventions can provide sustained support and overcome challenges associated with participating in DSCE, offering the potential for delivery in multiple locations at convenient times (15). Furthermore, certain mobile applications (apps) can assist physicians in determining a patient's blood sugar level, modifying the patient's lifestyle, enhancing blood sugar regulation, optimizing the patient's treatment plan, and performing individualized diabetes care, all of which can lower medical expenses (16). Through mHealth systems, patients with type 2 diabetes are encouraged to engage in physical activity and maintain a nutritious diet. However, the effect of apps on mHealth interventions remains unclear due to heterogeneous interventions and varying follow-up durations. Few studies have reported significant improvement in disease control when measuring hemoglobin A1c (HbA1c) levels (17).
The purpose of this study was to conduct a pragmatic, randomized controlled trial to determine the effects of a mobile health diabetes self-care program on HbA1c, FBS, and self-care in older adults with type 2 diabetes. We hypothesized that this mobile app would improve patient self-care and that patients using the app would ultimately have better HbA1c and FBS levels compared to controls.
2. Objectives
We have developed a mobile app called "Idia", which stands for competent and continuous management of diabetes self-care. This app has been meticulously designed to support and enhance diabetes self-care for individuals with diabetes. We conducted a 12-week randomized controlled trial to determine the effect of a mobile health diabetes self-care program on HbA1c, fasting blood sugar (FBS), and self-care in older adults with type 2 diabetes.
3. Methods
3.1. Study Design
A randomized clinical trial (RCT) was conducted over three months at the specialized Mostafavian Diabetes Clinic in Sari, Iran, by the nursing and aging research team of Mazandaran University. This study is part of a thesis project evaluating the effect of mobile phone-based education on self-care behavior and glycemic control in older adults with type 2 diabetes, conducted from May 2023 to August 2023. In this study, the intervention group used a mobile device app, while the control group had access to a printed booklet and received usual care, both containing content related to the care of older adults with type 2 diabetes.
3.2. Study Sample
Participants were eligible for inclusion in the study if they met the following criteria: Over 60 years old; ability to communicate and answer questions; informed consent; access to and ability to use a smartphone; access to the internet; ability to read the Persian language (self-reported); no prior use of self-care apps before the start of the study; and a diagnosis of diabetes by a physician at least 6 months prior. Exclusion criteria included failure to record information on the app for one week; participation in fewer than three teaching units; acute illness or hospitalization; loss of mobile phone and inability to replace it; withdrawal or unwillingness to continue participating in the study; or death of the patient.
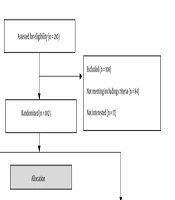
The sample size was based on the study by Rahnavard et al., with an error rate of 5% and a test power of 80% (18). Forty-six participants were calculated for each group. Finally, with an expected attrition rate of 10% after the pre-and post-intervention periods, 51 samples were collected in each group, totaling 102 samples. Eligibility was assessed for 210 patients, of whom 108 were excluded; 80 participants did not meet the inclusion criteria, and 17 declined to participate in the study. We reviewed the inclusion and exclusion criteria for the remaining 102 participants. Self-signed written informed consent was obtained from all participants.
A total of 102 participants were enrolled in the study and were randomly assigned to the intervention or control group using a random allocation program (https://www.randomizer.org). After division into two groups, 12 participants were eliminated. In the intervention group, 3 participants were lost to follow-up, 2 did not participate in the post-test, and 1 declined to participate in the study. In the control group, 4 participants could not be followed up, 1 did not take part in the post-test, and 1 refused to participate in the study. Therefore, 90 participants completed the study.
As this is an RCT using two educational technologies as an intervention, participants were blinded only to the research hypotheses and not to the two interventions used. The statistician was blinded to randomization, type of educational technology, and data analysis before and after the intervention. Blinding prevents bias on the part of the lead researcher and prevents differentiated attention to the intervention group (Figure 1).
3.3. Procedures
At the beginning of the study, older adults who met the research criteria and signed the informed consent completed a Sociodemographic and Self-care Diabetes Scale (SDSCA) Questionnaire. Additionally, biochemical blood samples, including HbA1c and FBS, were collected. During the clinical trial, participants in the experimental group manually recorded their diabetes self-care data (nutrition information, blood sugar levels, insulin and medications, physical activity, and emotions) on their mobile phones and attended eight courses per month on the Idia chat page app. Furthermore, older adults in the intervention group received 30 to 60 minutes of training on how to use the app. The researcher had access to all information collected from participants through the Idia app database and indirectly influenced the patients' treatment and lifestyle programs.
Participants in the control group received an educational booklet previously developed and validated by experts, covering the same topics as the app, and received usual support. During this meeting, the contents of the brochure were typically presented for 30 to 60 minutes. After the presentation, the researcher informed participants that they should take the booklet with them to read and receive usual care for one month. At the end of the study, both groups repeated the pretest assessments and laboratory tests to compare pre- and post-intervention measurements between and within groups, thereby evaluating the effect of its use in older adults. After the implementation of the protocol, both the intervention group and the control group had access to the app and printed brochure.
The main topics of this program were based on the SDSCA subscales and included a healthy eating plan, controlling glucose levels, engaging in regular physical activity, adhering to medication treatments, and promoting behaviors aimed at reducing the risk of complications (19). In this context, the app was prepared with the aforementioned topics. The learning booklet was created with the same content as the app. To prepare the content of the topics covered in these educational tools, a bibliographic review of global references on primary care interventions for older adults with diabetes was conducted (20, 21). The content was validated by an endocrinologist and faculty members from Nasibeh Nursing and Midwifery School.
3.4. Research Tools
3.4.1. Demographic Questionnaire
This questionnaire includes demographic factors such as age, gender, marital status, life companions, education level, employment status, income status, cognitive status, weight, height, and Body Mass Index (BMI). It also encompasses clinical factors, including chronic comorbid diseases, type of chronic disease, duration of disease, and type of medication used.
3.4.2. Summary of Diabetes Self-care Activities
This questionnaire, developed by Toobert et al. (22), aims to evaluate the self-care activities of individuals with diabetes over the past 7 days. It consists of 15 questions covering 6 areas: Diet, physical activity, blood sugar measurement, regular medication intake, and smoking. Each question is assigned a score from 0 to 7, indicating the number of days in the last week that a person engaged in self-care behavior. The total score ranges from 0 to 99, with a higher score indicating better self-care activities. The content validity of the questionnaire was confirmed with an average value of 84.9, and the reliability was 0.78.
3.4.3. Hemoglobin A1c
The HbA1c test was conducted at the Touba Laboratory in Sari using an ideal future detection kit and a turbidimetric method, which involves antigen-antibody interaction for the direct determination of HbA1c.
3.4.4. Fasting Blood Sugar
The FBS measurement was carried out using the Iranian-made DIABAN SMM1000 blood glucose meter.
3.5. Data Collection Process
The questionnaires were completed in approximately 45 minutes in the presence of one of the researchers. The researchers were available to address any questions, explain the method of completing the scales, and ensure that all questions were answered. They also emphasized the importance of providing honest responses and assured participants of the anonymity and confidentiality of the information contained in the questionnaire. Fasting blood sugar was measured weekly by the researcher during the one-month intervention period. Additionally, HbA1c tests were performed again for both groups three months after the start of the intervention.
3.6. Application
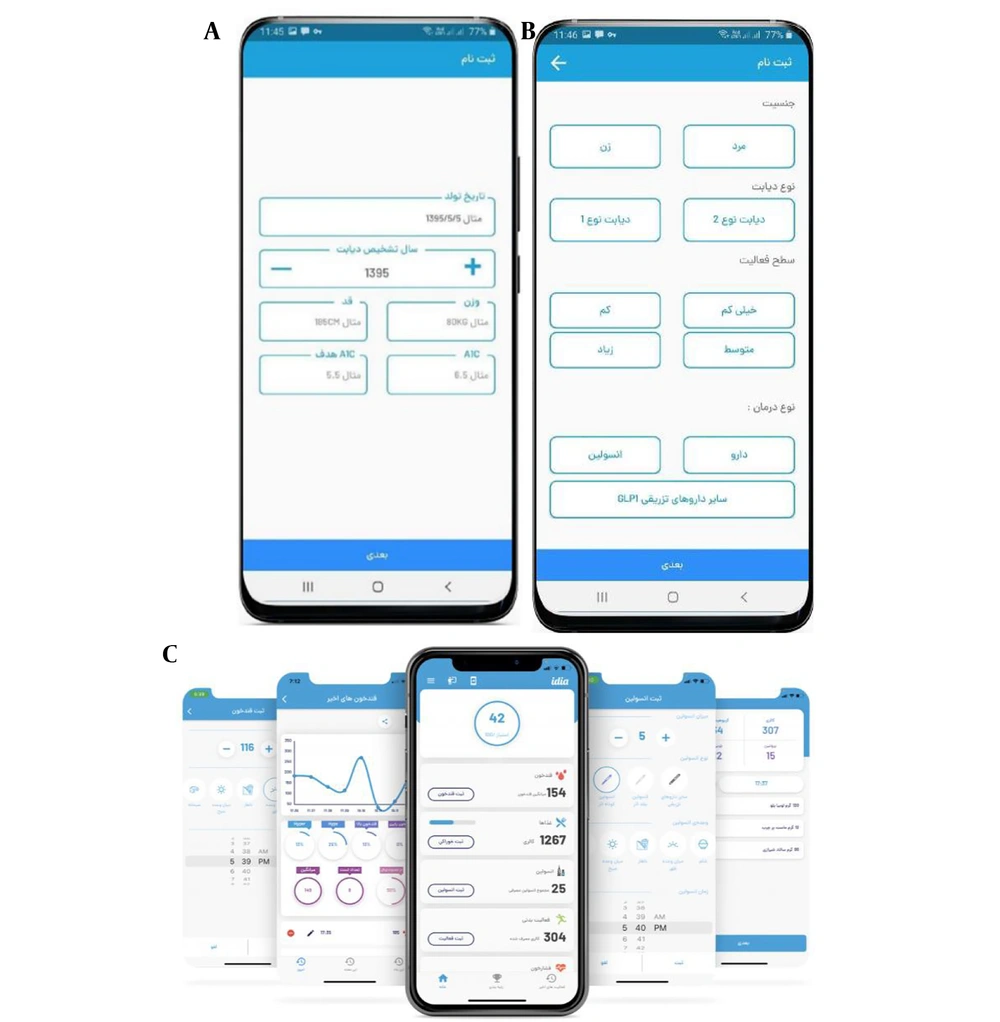
The app called Idia was created in 2020 and currently has 20,000 active users in Iran. It covers various areas of diabetes care, including nutrition, exercise, measurement tracking, medication logging, emotion tracking, and education. Users receive customized plans based on their personal information. The app calculates the appropriate caloric intake for each individual and sets nutritional goals. The physical activity section displays the number of steps, distance, duration of the activity, and the number of calories burned. Additionally, users are encouraged to use the app as a personal diabetes diary to log their blood sugar and glycated hemoglobin levels, weight, and track medications (insulin and others). The educational section of the software provides users with valuable information about diabetes.
One of the strengths of the Idia software is its scoring system, where users receive points based on key criteria related to blood sugar control. This assessment helps individuals with diabetes to understand their daily conditions more accurately and effectively. Before using the app, users provide information such as type of diabetes, type of treatment, initial and target glycosylated hemoglobin level, physical activity, gender, age, height, and weight. All information is displayed on the main home screen. Users can also create daily, weekly, and monthly blood glucose reports in graphs to share with specialists when needed (Figure 2).
3.7. Outcomes and Measures
Primary outcomes were changes in participants' diabetes self-care, assessed using the SDSCA Questionnaire at week 4 and week 12, compared to baseline. Secondary outcomes included changes in laboratory tests, specifically measurements of fasting plasma glucose and HbA1c.
3.8. Data Analysis
Statistical analyses were performed using SPSS version 26. The normality of the variables was assessed using the Kolmogorov-Smirnov test, which indicated that the distribution of the variables was normal only for BMI and total self-care score. The homogeneity of demographic and clinical characteristics and study variables between the two groups was analyzed using the t-test, Mann-Whitney U test, or chi-square test. Patient characteristics, baseline HbA1c, and FBS values were summarized using descriptive statistics, including means and standard deviations for quantitative variables and proportions for qualitative variables. The difference between the pre-test and post-test within each group was analyzed using the Friedman test. Differences in post-test and pre-test scores between the two groups were identified using the chi-square test, Fisher's exact test, and the Mann-Whitney U test. All tests were performed at a significance level of 0.05.
3.9. Ethical Considerations
This study adhered to the principles of the Declaration of Helsinki. The study protocol was approved by the Mazandaran University of Medical Sciences Ethics Committee (IR.MAZUMS.REC.1402.193). Additionally, the study is registered in the Iranian Clinical Trials Registry (IRCT) under the number IRCT20170611034454N5. All participants signed a written informed consent form. Confidentiality of information was maintained, and participants were allowed to withdraw from the study at any stage.
4. Results
The results on participants' demographic characteristics indicated that the mean and standard deviation of participants' ages in the intervention and control groups were 63.2 ± 2.84 years and 62.2 ± 2.22 years, respectively. In terms of gender distribution, 45 participants (50%) were male and 45 (50%) were female. Statistical tests, including the Mann-Whitney test for quantitative variables and chi-square and Fisher's exact tests for qualitative variables, demonstrated that the intervention and control groups were similar in demographic characteristics, with no significant differences initially observed between the two groups (P > 0.05). The only significant difference noted was in income level, with a higher proportion of individuals with insufficient income in the intervention group compared to the control group (P = 0.11) (Table 1).
| Variables | Control Group (n = 45) | Intervention Group (n = 45) | Overall | P-Value |
|---|---|---|---|---|
| Gender | 0.527 b | |||
| Male | 21 (46.7) | 24 (53.3) | 45 (50) | |
| Female | 24 (53.3) | 21 (46.7) | 45 (50) | |
| Marital status | > 0.999 b | |||
| Single or widowed | 5 (11.1) | 5 (11.1) | 10 (11.1) | |
| Married | 40 (88.9) | 40 (88.9) | 80 (88.9) | |
| Education | > 0.999 b | |||
| High school or less | 24 (53.3) | 24 (53.3) | 48 (53.3) | |
| Diploma | 16 (35.6) | 16 (35.6) | 32 (35.6) | |
| University degree | 5 (11.1) | 5 (11.1) | 10 (11.1) | |
| Household income | 0.011 b | |||
| Insufficient | 26 (57.8) | 37 (82.2) | 63 (70) | |
| Sufficient | 19 (42.2) | 8 (17.8) | 27 (30) | |
| Time since diabetes diagnosis | > 0.999 c | |||
| 6 - 12 months | 5 (11.1) | 4 (8.9) | 9 (10) | |
| > 12 months | 40 (88.9) | 41 (91.1) | 81 (90) | |
| Comorbidities | 0.250 b | |||
| Yes | 29 (64.4) | 34 (75.6) | 63 (70) | |
| No | 16 (35.6) | 11 (24.4) | 27 (30) | |
| Comorbidities | 0.255 c | |||
| No comorbidities | 16 (35.6) | 11 (24.4) | 27 (30) | |
| Heart diseases | 21 (46.7) | 29 (64.4) | 50 (55.6) | |
| Kidney disease | 7 (15.6) | 3 (6.7) | 10 (11.1) | |
| Cancer | 1 (2.2) | 2 (4.4) | 3 (3.3) | |
| Diabetesmedications | 0.317 c | |||
| Oral | 29 (64.4) | 35 (77.8) | 64 (71.1) | |
| Insulin | 11 (24.4) | 8 (17.8) | 19 (21.1) | |
| Both | 5 (11.1) | 2 (4.4) | 7 (7.8) | |
| BMI (kg/m2) | 29.4 ± 3.37 | 28.4 ± 2.63 | 28.9 ± 3.05 | 0.129 d |
Abbreviation: BMI, Body Mass Index.
a Values are expressed as No. (%) or mean ± SD.
b Chi-squared test.
c Fisher’s exact test.
d Mann-Whitney U test.
According to the results presented in Table 2, the mean values of HbA1c and FBS in patients from both groups before the intervention showed no significant difference (P > 0.05). However, after the intervention, HbA1c levels decreased from 7.25% to 6.8% (P < 0.001) in the intervention group and from 7.24% to 7.19% (P < 0.001) in the control group. Similarly, FBS values decreased from 171 mg/dL to 122 mg/dL (P < 0.001) in the experimental group and from 181 mg/dL to 145 mg/dL (P < 0.001) in the control group. Although both groups exhibited these changes, the differences were more pronounced in the experimental group compared to the control group (Tables 2 and 3).
Abbreviations: CI, confidence interval; HbA1c, hemoglobin A1c.
a Values are expressed as mean ± SD.
b Regression coefficient.
c Generalized estimating equations test.
Abbreviations: CI, confidence interval; FBS, fasting blood sugar.
a Values are expressed as mean ± SD.
b Regression coefficient.
c Generalized estimating equations test.
The mean self-care score among patients in the two groups before the intervention was not statistically significant (P > 0.05). However, after the intervention, the score was significantly higher in the intervention group compared to the control group (P < 0.001). Specifically, the mean change in self-care was an increase of 15.1 units for patients in the intervention group and an increase of 1.1 units for patients in the control group. The increase in the experimental group was more pronounced compared to the control group (Table 4).
| Variables | Baseline | First Month | Third Month | B (95% CI) | P-Value b |
|---|---|---|---|---|---|
| Total self-care activities | 0.282 c (0.113, 0.452) | 0.001 | |||
| Control group | 28.9 ± 8.98 | 30 ± 7.98 | 29.9 ± 7.83 | ||
| Intervention group | 29.9 ± 5.57 | 45 ± 5.4 | 43.2 ± 4.59 | ||
| Diet | 0.119 c (0.006, 0.233) | 0.039 | |||
| Control group | 12.76 ± 3.8 | 12.89 ± 3.61 | 12.78 ± 3.55 | ||
| Intervention group | 12.7 ± 3.18 | 15.98 ± 2.74 | 15.49 ± 2.27 | ||
| Exercise | 0.309 c (0.106, 0.512) | 0.003 | |||
| Control group | 3.56 ± 1.62 | 3.73 ± 1.62 | 3.73 ± 1.62 | ||
| Intervention group | 3.82 ± 1.76 | 5.96 ± 0.85 | 5.82 ± 1.01 | ||
| Blood sugar testing | 0.046 c (-0.106, 0.197) | 0.555 | |||
| Control group | 2.40 ± 2.18 | 2.78 ± 1.94 | 2.78 ± 1.94 | ||
| Intervention group | 2.24 ± 1.89 | 3.38 ± 1.53 | 2.56 ± 1.25 | ||
| Medications | 0.307 c (0.11, 0.504) | 0.002 | |||
| Control group | 4.04 ± 2.29 | 4.13 ± 2.17 | 4.11 ± 2.17 | ||
| Intervention group | 4.13 ± 2.03 | 6.36 ± 1.25 | 6.22 ± 1.28 | ||
| Foot care | 0.589 c (0.287, 0.891) | < 0.001 | |||
| Control group | 6.04 ± 4.39 | 6.40 ± 4.02 | 6.40 ± 4.02 | ||
| Intervention group | 6.16 ± 3.08 | 13.2 ± 3.18 | 13 ± 3.02 |
Abrreviation: CI, confidence interval.
a Values are expressed as mean ± SD.
b Generalized estimating equations test.
c Regression coefficient.
5. Discussion
Due to the increasing burden of noncommunicable diseases in developing countries, particularly diabetes, it is becoming increasingly important to prevent and control such diseases to avoid associated complications. On the other hand, given the critical role of lifestyle in the development and progression of diabetes, the importance of lifestyle modification in these patients is increasingly highlighted (23). Therefore, the aim of the present study was to determine the effects of software-based Idia training on self-care behavior and blood glucose levels in older adults with type 2 diabetes.
The results showed that one month after the intervention, the average self-care behavior score of older adults in the intervention group increased significantly compared to the control group. Various studies in different countries have demonstrated the positive effects of digital software on the self-care behavior of individuals with diabetes (24, 25). According to our results, the mean self-care score among patients in the two groups before the intervention was not statistically significant. However, one and three months after the start of the intervention, the mean self-care score in the intervention group was significantly higher than in the control group. Although one month after the start of the study, the mean self-care score increased in both groups and decreased three months later, the rate of increase was much higher in the intervention group than in the control group. Therefore, it can be concluded that education improved self-care among participants in both groups, but software-based education had a greater positive impact. Studies have shown that innovative technologies for patient education and monitoring achieve better results compared to traditional education methods (17, 26).
Based on the results of the current study, participants in the intervention group adhered to recommendations for adequate nutrition, increased physical activity, regular medication, and foot care significantly more than the control group. However, the average BMI decreased slightly in both groups after the procedure. Regarding this finding, it was expected that the intervention group would experience greater weight loss, as some studies have shown significant differences in BMI values in diabetes self-care post-intervention. This contradiction could likely be due to the fact that the older adult population studied may experience changes such as decreased metabolism, hypothyroidism, and hormonal changes that may impact weight changes (25, 27, 28). Additionally, stronger motivations may be needed to change habits, such as exercising and quitting smoking.
Regarding the glycemic control subscale, no significant difference was observed between the two groups, and the average self-care score in the blood glucose test after the intervention increased slightly in both groups. In this context, it can be noted that in most developing countries, there is a significant gap between practical recommendations and the care provided, resulting in poor glycemic control (29).
One of the study's other findings was a gradual decline in average FBS in both the intervention and control groups. Before the intervention until one week after the start of the intervention, there was no statistically significant difference in FBS in both groups, but from the third week after the start of the intervention, the average FBS in the intervention group was significantly lower than in the control group. Although this decrease was observed in both groups, the decrease in fasting blood glucose was greater in the intervention group. The results of other studies also confirm that the reduction in FBS was significantly greater in the intervention group compared to the control group (30, 31).
The results of the present study showed a significant difference in the reduction of HbA1c in the intervention group compared to the control group. Consistent with our study, Azizi et al. (32) demonstrated that the mean serum HbA1c levels after the intervention were significantly lower in the intervention group than in the control group. Although the intervention reduced HbA1c, it did not achieve a significant reduction compared with previous programs (33), and there are several likely reasons for this. First, the baseline HbA1c level was not an inclusion criterion. The significant difference by baseline HbA1c observed in the subgroup analysis suggests that the intervention may have significant benefits if the study had targeted only those individuals with HbA1c above target. Second, similar to some previous digital health trials (34), there was a reduction in HbA1c among participants in the control arm. This could be due to the Hawthorne effect, where participants sufficiently motivated to be involved in the trial are likely to seek better self-management through other options when they were not allocated to the active intervention.
Lifestyle modifications, diabetes education, and self-care are some potential mechanisms that lead to improved glycemic control. Self-care is implemented in the Idia app through medication, meal, and glucose reminders. In addition, physical activity support and individual training plans in the gym and at home are available to users. Finally, Idia provides users with appropriate meal plans that are created according to the users' individual needs.
One of the strengths of our study was that it was a stratified RCT, ensuring similar baseline characteristics between groups. There were also some limitations. First, due to the nature of the intervention, we were unable to blind participants and those providing clinical measurements to study assignment. Without the ability to blind participants, self-report bias and Hawthorne effects can occur. Second, we selected participants from one health center, which did not include all diabetic patients in the city; therefore, the results cannot be representative of all diabetic patients in the city. Third, although approximately 80% of the intervention group reported overall satisfaction with the app and were neutral or positive about its support in diabetes management, they also emphasized the need for a more user-friendly experience and a lighter version of the app.
5.1. Conclusions
In conclusion, this study suggests that the implementation of an educational intervention via a mobile app results in a significant reduction in serum FBS and HbA1c levels, as well as a significant increase in average self-care. While further research involving larger and more diverse populations is required to validate these findings, the results provide valuable evidence for the potential efficacy of utilizing such mobile apps to enhance the overall health and well-being of individuals living with type 2 diabetes mellitus (T2DM).