1. Background
Diabetic foot ulcers (DFUs) are among the prevalent complications in diabetic patients, with a high prevalence in most communities (1, 2). It is estimated that the overall mortality rate for DFUs is significant, with almost 50% of patients dying within five years. The primary causes of death are cardiovascular disease and infections (3). As a result, researchers and healthcare professionals are continuously exploring innovative approaches to improve the healing process of DFUs (4). Ozone therapy, known for its potent disinfectant and oxidative properties, is acknowledged as an effective approach for managing and treating DFUs (5). This therapy promotes glucose utilization at the cellular level, enhances protein metabolism, and facilitates the oxidation of unsaturated lipids, all of which concurrently activate repair mechanisms (6). Researchers observed that, when compared to standard control therapies for DFUs, both monotherapy and combination ozone therapy significantly improved wound healing outcomes (7).
Olive oil represents another therapeutic option for managing DFUs, as it enhances wound healing by promoting blood circulation to the affected tissue and reducing inflammation surrounding the wound (8). Several studies examining the impact of olive oil on DFU treatment have recognized that this intervention significantly improves wound grade, drainage, and overall healing in DFUs (8, 9). Furthermore, a recent systematic review and meta-analysis concluded that olive oil can facilitate the healing process of DFUs (10). The addition of ozone enhances the properties of oils, including olive oil. In ozonated olive oil, the formation of carbon-carbon double bonds in unsaturated fatty acids enhances biological activities, thereby augmenting the anti-inflammatory properties of this compound (11, 12).
A review of 28 articles revealed that ozonated olive extract is effective in promoting wound healing. Its benefits include reduced microbial infection, debridement, modulation of inflammation, angiogenesis stimulation, and enhanced oxygen metabolism, which collectively contribute to improved wound closure (13). Some clinical trials conducted evaluated the efficacy of ozonated olive ointment in treating DFUs and found it to be more effective than conventional solutions across all grades of DFUs (14, 15). Given the rapid expansion of clinical trials and the quick administration of O3 therapy, it has the potential to become a leading treatment for DFUs. While promising clinical evidence exists, further research is needed to fully comprehend the role of O3 in DFUs (16).
Despite the growing body of evidence supporting the benefits of ozonated olive oil in wound healing, several gaps remain in the current literature. Previous studies have primarily focused on the general antimicrobial and anti-inflammatory properties of ozonated oils, but few have specifically explored the efficacy of nurse-led interventions using ozonated olive oil in a clinical setting, particularly for DFUs (12, 17). Additionally, while many studies have demonstrated positive outcomes in small sample sizes or in vitro, there is a lack of large-scale, randomized controlled trials that directly compare ozonated olive oil with conventional treatments across different severities of DFUs (11).
While ozone therapy has demonstrated promise in wound healing, especially for DFUs, direct comparative studies evaluating the efficacy of ozone-infused oils against other ozone therapy modalities are scarce. Rigorous, large-scale clinical trials are imperative to definitively establish the superiority of one approach over another. Despite the significant potential of ozone and olive oil in treating DFUs, further research is needed to explore their efficacy across diverse populations. The effectiveness and safety of ozone therapy for chronic wounds or ulcers remain uncertain, necessitating more robust investigations to address this knowledge gap (7).
2. Objectives
This study aims to investigate the effect of ozonated olive oil on the healing of DFUs and compare its effectiveness with standard treatments to evaluate its potential as a new therapeutic option for enhancing wound healing.
3. Methods
3.1. Design
This study was conducted as a prospective, randomized, controlled trial with a parallel-group design from 20 July to 1 September 2023 at a private outpatient clinic. It was registered in the clinical trial database (IRCT20240509061721N2). The research adhered to the World Medical Association's code of ethics (Declaration of Helsinki) for experiments involving human subjects. The study followed the consolidated standards of reporting trials (CONSORT) guidelines (18).
3.2. Participants
Participants eligible for this clinical trial were required to have a confirmed diagnosis of type 1 or type 2 diabetes mellitus (which was based on the patients' medical records or the diagnosis of the physician) and be between the ages of 18 and 75 years. Another inclusion criteria was suffering from at least one diabetic foot ulcer that had persisted for more than 1 week; the decision to include participants with ulcers that had lasted at least one week was based on the understanding that DFUs typically begin to show early signs of chronicity after this time frame, making it a suitable threshold for evaluating the effectiveness of treatments aimed at promoting wound healing (17). Additionally, grade 1 or grade 2 according to the Wagner’s classification system was acceptable. Also, participants had to be capable of providing informed consent and adhering to the study protocol.
Exclusion criteria included pregnancy or lactation, active infections or systemic diseases that could interfere with wound healing (e.g., osteomyelitis or cellulitis), a history of malignancy or current cancer diagnosis affecting the lower limbs, recent surgical procedures or trauma to the foot, known allergy or hypersensitivity to ozone or any component of the ozonated olive oil, and conditions that might interfere with study outcomes, such as severe renal or hepatic dysfunction. Participants who were unlikely to comply with the study protocol or follow-up visits due to socio-economic or psychological reasons were also excluded.
The minimum sample size is determined based on a similar study (19) using the formula provided. Therefore, accounting for a 15% dropout rate in each group, a total of 70 participants, with 35 in each group, is deemed appropriate for this study.
3.3. Randomization and Blinding
Participants were recruited through a systematic sampling process prior to random assignment. Eligible individuals were identified based on predefined inclusion and exclusion criteria, and those who provided informed consent were invited to participate in the study. The recruitment process was conducted to ensure a representative sample of participants fitting the study's criteria. After obtaining informed consent, participants were screened for eligibility, and only those meeting the criteria were included in the final sample.
Participants were randomly assigned to either a control group or an intervention group in a 1:1 ratio using a computer-generated randomization algorithm. The randomization process was conducted via a web-based platform (http://www.randomization.com) to ensure unbiased allocation. The sequence of randomization was generated by the platform, and the assignments were concealed through a process known as "allocation concealment". This was achieved by preparing sealed, opaque envelopes containing the study protocols for each group. The envelopes were sequentially numbered according to the randomization sequence and were opened only at the time of enrollment for each participant, ensuring that neither the primary researcher nor the co-researcher, who were responsible for participant enrollment and wound assessment, had prior knowledge of the group assignment.
To ensure that the allocation sequence remained concealed, the randomization schedule was securely stored in a locked cabinet within the department, with access restricted solely to the principal investigator. Identifiers assigned to participants during the randomization process were random numbers, preserving confidentiality. Importantly, no patient-specific identifiers, such as hospital registration numbers, were used to maintain blinding and further conceal the allocation process.
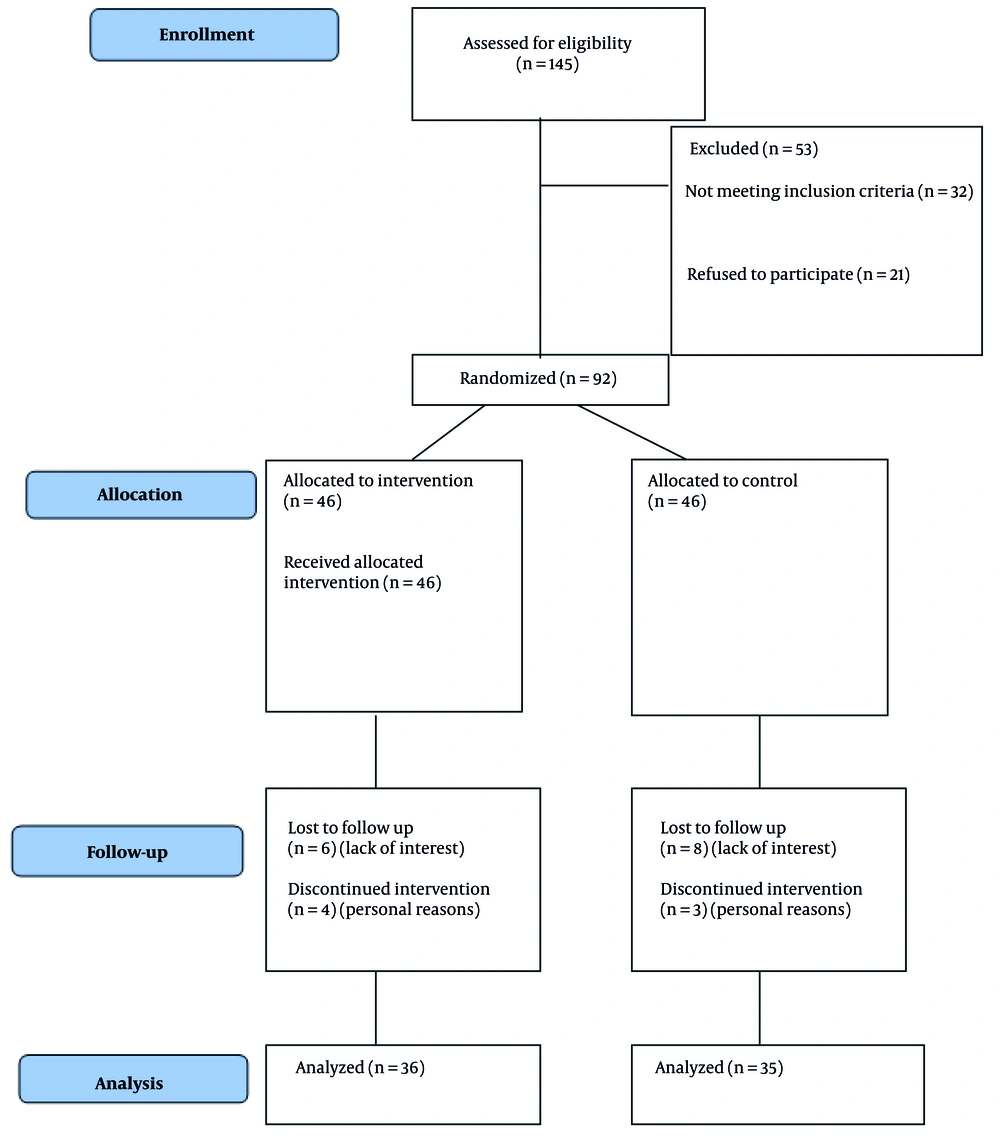
Single blinding was implemented for the assessors involved in wound evaluation, data collection, and analysis. While it was not feasible to blind the participants and the staff applying the treatment due to the distinct appearance and consistency of the intervention (e.g., olive oil), strict blinding was maintained during the data collection phase. To minimize any bias during wound assessments, staff members were instructed to thoroughly cleanse the wound bed with normal saline before performing any evaluations, ensuring that assessors were unaware of the treatment group to which the participant had been assigned. Figure 1 shows the process of study participants based on the CONSORT flowchart.
3.4. Tools
The primary outcomes assessed and compared demographic variables, site of ulcer, grade of ulcer, vascular status of ulcer, neuropathy status of ulcer, adverse degree at the end of the study, status of ulcer healing at the end of the study, mean wound grade and color, surrounding tissues, drainage, and overall wound condition. The data collection tools utilized in this study comprised several key components: A demographic and clinical characteristics form, the Wagner wound classification system (20), and a checklist for evaluating the healing progress of DFUs.
The demographic form captured information on age, gender, weight, height, education status, marital status, job status, mobility status, smoking, drug abuse, and history of DFUs. This form was completed by the study participants or their caregivers and verified by the research team.
The Wagner wound classification system was used to categorize ulcers based on their depth and the presence of complications like osteomyelitis or gangrene. The system uses the following grades:
- Grade 0: Pre-or post-ulcerative lesion.
- Grade 1: Partial or full-thickness ulcer.
- Grade 2: Ulcer probing to tendon or capsule.
- Grade 3: Deep ulcer with osteitis.
- Grade 4: Partial foot gangrene.
- Grade 5: Whole foot gangrene.
The Wagner classification was evaluated by trained clinicians who had experience in wound care and diabetic foot management. To ensure reliability, a subset of ulcers was independently assessed by two clinicians, and the inter-rater reliability was calculated using Cohen’s Kappa statistic, which demonstrated strong agreement (κ = 0.85) (21).
The ulcer healing assessment scale was used weekly to evaluate four parameters: Color, surrounding tissue condition, drainage, and overall ulcer status. Each parameter was scored on a scale of 0 to 100, where higher scores indicated better healing progress. Specifically, color was rated based on changes in the appearance of the wound bed (from necrotic to granulating tissue), surrounding tissue condition was assessed for signs of inflammation or infection, drainage was quantified based on volume and consistency, and the overall status reflected a comprehensive evaluation of the ulcer's healing stage.
The Ulcer Healing Assessment Scale has also been validated for reliability and validity in Iran. The reliability coefficient was found to be 0.90, and the validity coefficient was 0.87. These values demonstrate a high level of consistency and accuracy in evaluating ulcer healing based on the four parameters (color, surrounding tissue condition, drainage, and overall status) in the Iranian context (22, 23).
3.5. Intervention
During the initial visit, patients were provided with a detailed explanation of the project’s objectives, intervention methods, and duration, along with comprehensive education on DFU complications. Additionally, data on the general and clinical characteristics of the participants were collected. A single trained research team member evaluated DFUs for all enrolled patients. In the first session, patients were instructed on the correct technique for dressing their wounds. An infectious disease specialist, alongside a trained research member, was responsible for infection control, emphasizing a multidisciplinary approach to infection management.
Initially, the patient's wound condition was assessed using relevant tools and practical criteria, such as the Wagner classification system. Based on the wound's status, a wound bed preparation (WBP) was carried out following the local wound care TIMES protocol (tissue nonviable, inflammation or infection, moisture, exudate, and surrounding skin) (24). The TIMES protocol was applied in accordance with the clinical characteristics of the wound.
Both groups received standard treatments and care for diabetes and foot ulcer management. For the control group, standard care involved irrigating and cleaning both the central and peripheral dimensions of the ulcers with sterile normal saline, allowing the area to dry, and then applying a sterile gauze dressing. For the intervention group, the ulcer surface was first covered with ozonated olive oil using a sterile syringe. The herbal wound healing medication, ozone, prescribed to the intervention group in the study, is a product based on technical expertise from Feedar Pharmad Sadir Gharn Company, as its products were available on the market to meet the needs of the current study. It is formulated using organic extra virgin olive oil, which is certified organic by the European Union, has health certification from the Iranian Food and Drug Administration, and complies with good manufacturing practices (GMP).
The ozonated olive oil used in the study was prepared by infusing organic extra virgin olive oil with ozone gas in a controlled process. The olive oil was placed in a closed chamber, where ozone gas was introduced at a specific concentration and temperature to ensure proper ozonation. This process enhances the oil's antimicrobial and healing properties, making it effective for wound care. The resulting ozonated olive oil was then packaged for use in the intervention group.
Oral antibiotics (ciprofloxacin twice a day and clindamycin three times a day based on the prescription of the physician) were administered to both groups according to the condition of the ulcers and clinical assessment for mild to moderate infected wounds that exhibited at least two signs or symptoms of inflammation — such as redness, warmth, tenderness, pain, and swelling — or purulent discharge.
The training and dressing procedures were carried out by trained research team members, including a dedicated wound care specialist and, in some cases, a nurse. Patients either visited a private clinic for the treatment or had the intervention performed at their homes, depending on their individual needs and preferences. The intervention was carried out at either the private clinic or in the patient's home, as determined by their clinical condition and the logistics of their care.
The ozonated olive oil was applied directly to the wound surface, with approximately 5 mL of the solution used for each application. The application was performed every two days for the duration of the study (4 weeks). This consistent application was done to ensure optimal contact with the wound and to facilitate the healing process. The amount used was standardized to maintain consistency across all participants in the intervention group.
At the end of each week, for a continuous period of four weeks, the aforementioned tools were meticulously completed for each participant by trained research team members. To minimize potential biases, several strategies were employed. First, all research team members received standardized training to ensure consistency in data collection and evaluation. Additionally, to reduce observer bias, a subset of assessments was independently reviewed by a second clinician, and inter-rater reliability was assessed. The study also incorporated randomization in participant selection and blinded assessment of healing progress, where the clinicians evaluating ulcer healing were unaware of the participants’ previous scores or any group assignment.
3.6. Statistical Analysis
Following data collection, the analysis was divided into two main sections: Descriptive and analytical statistics. Initially, the distribution of quantitative data was assessed for normality using skewness and kurtosis statistics. In the descriptive statistics section, quantitative data were summarized with the mean and standard deviation, while qualitative data were described using frequencies. For the analytical statistics, the chi-square test was employed to analyze qualitative data, and the independent t-test was used for comparing means between groups. Additionally, if necessary, Fisher’s exact test and repeated measures ANOVA were used. A significance level of P ≤ 0.05 was considered significant. SPSS Statistics version 22 was used for the analysis.
3.7. Ethical Considerations
Ethical considerations in this study were rigorously followed to ensure the protection of participants' rights and safety. Informed consent was obtained from all participants after they were fully briefed on the study's purpose, procedures, potential risks, and benefits. They were informed of their right to withdraw at any time without consequence. Confidentiality was strictly maintained throughout the study by anonymizing personal data and storing it securely. Additionally, the study received approval from the Ethics Committee of Hormozgan University of Medical Sciences (IR.HUMS.REC.1403.128).
Participant safety was prioritized, with continuous monitoring for any adverse effects from the ozonated olive oil intervention. In the event of any serious side effects, participants were withdrawn from the study and provided with appropriate medical care. Participation was entirely voluntary, and individuals could withdraw at any time without affecting their treatment. The study followed principles of beneficence and non-maleficence, ensuring that the intervention was both safe and potentially beneficial for participants. Ethical standards were upheld throughout the study to guarantee the well-being and confidentiality of all participants.
4. Results
In this study, 36 participants in the intervention group and 35 participants in the control group were analyzed. At baseline, the two groups showed some demographic and clinical differences. The intervention group was significantly younger (mean age 58.33 years) compared to the control group (mean age 63.80 years; P = 0.00). Insulin usage duration was also significantly shorter in the intervention group (2.38 days vs. 5.13 days; P = 0.01). Other demographic variables, including weight, height, marital status, education, job status, smoking, drug abuse, and mobility, showed no significant differences (all P > 0.05; Table 1).
| Variables and Categories | Intervention (n = 36) | Control (n = 35) | P-Value |
|---|---|---|---|
| Age (y) | 58.33 ± 6.13 | 63.80 ± 6.53 | 0.00 |
| Weight (kg) | 73.64 ± 8.68 | 76.74 ± 19.47 | 0.38 |
| Height (cm) | 171.56 ± 8.09 | 167.89 ± 17.97 | 0.26 |
| Insulin usage time (d) | 2.38 ± 1.45 | 5.13 ± 3.67 | 0.01 |
| Gender | 0.73 | ||
| Female | 22 (61.1) | 20 (57.1) | |
| Male | 14 (38.9) | 15 (42.9) | |
| Education status | 0.23 | ||
| Illiterate | 0 | 1 (2.9) | |
| Under diploma | 13 (36.1) | 10 (28.6) | |
| Diploma | 14 (38.9) | 11 (31.4) | |
| Associate | 5 (13.9) | 2 (5.7) | |
| Bachelor | 4 (11.1) | 9 (25.7) | |
| Master | 0 | 2 (5.7) | |
| Marital status | 0.64 | ||
| Single | 3 (8.6) | 2 (5.7) | |
| Married | 32 (91.4) | 33 (94.3) | |
| Job status | 0.24 | ||
| Employed | 3 (8.3) | 1 (2.9) | |
| Retired | 11 (30.6) | 17 (48.6) | |
| Housekeeper | 22 (61.1) | 17 (48.6) | |
| Mobility status | 0.09 | ||
| Normal | 33 (91.7) | 27 (77.1) | |
| Low | 3 (8.3) | 8 (22.9) | |
| Smoking | 0.27 | ||
| Yes | 9 (25) | 13 (37.1) | |
| No | 27 (75) | 22 (62.9) | |
| Drug abuse | 0.15 | ||
| Yes | 0 | 2 (5.7) | |
| No | 36 (100) | 33 (94.3) | |
| History of previous diabetic foot | 0.27 | ||
| Yes | 19 (55.9) | 13 (40.6) | |
| No | 15 (44.1) | 19 (59.4) | |
| Site of ulcer | 0.16 | ||
| Sole | 10 (28.6) | 9 (25) | |
| Heel | 16 (45.7) | 20 (55.6) | |
| Dorsum of the feet | 6 (17.1) | 1 (2.8) | |
| Toes | 3 (8.6) | 6 (16.7) | |
| Grade of ulcer | 0.00 | ||
| Wagner 1 | 11 (28.6) | 10 (28.5) | |
| Wagner 2 | 26 (74.3) | 25 (71.4) | |
| Vascular status | 0.11 | ||
| Normal | 0 | 3 (8.6) | |
| Abnormal | 36 (100) | 32 (91.4) | |
| Neuropathy status | 0.12 | ||
| Normal | 0 | 3 (8.6) | |
| Abnormal | 36 (100) | 32 (91.4) | |
| Dimension (cm²) | 4.42 ± 1.30 | 4.26 ± 1.36 | 0.61 |
| Depth (cm) | 2.58 ± 0.77 | 2.34 ± 1.03 | 0.26 |
| Time of wound formation (wk) | 0.66 ± 0.41 | 0.88 ± 0.69 | 0.11 |
| ABI | 0.71 ± 0.08 | 0.70 ± 0.10 | 0.57 |
Abbreviation: ABI, Ankle-Brachial Pressure Index.
a Values are expressed as No. (%) or mean ± SD.
Baseline wound characteristics were mostly similar between groups. The ulcer site distribution was not significantly different (most common site: Heel; P = 0.16). However, ulcer grade differed significantly, with the intervention group having more Wagner grade 1 ulcers (71.4%) compared to the control group (28.6%; P = 0.00). Vascular and neuropathy status were abnormal in both groups, but the differences were not significant (P = 0.11 and P = 0.12, respectively). Quantitative measures, such as wound dimensions, depth, and Ankle-Brachial Pressure Index, showed no significant differences (all P > 0.05; Table 1).
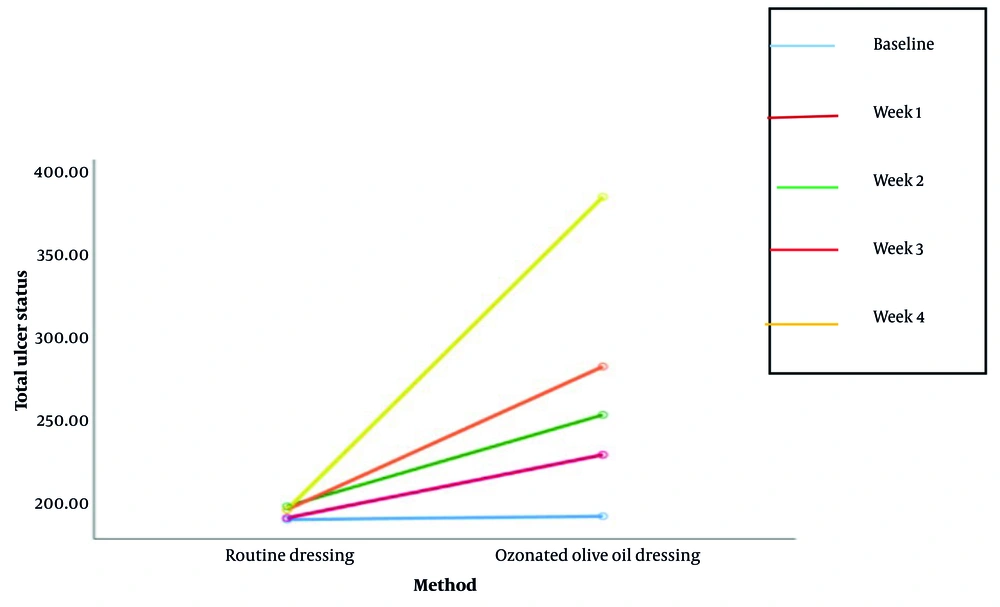
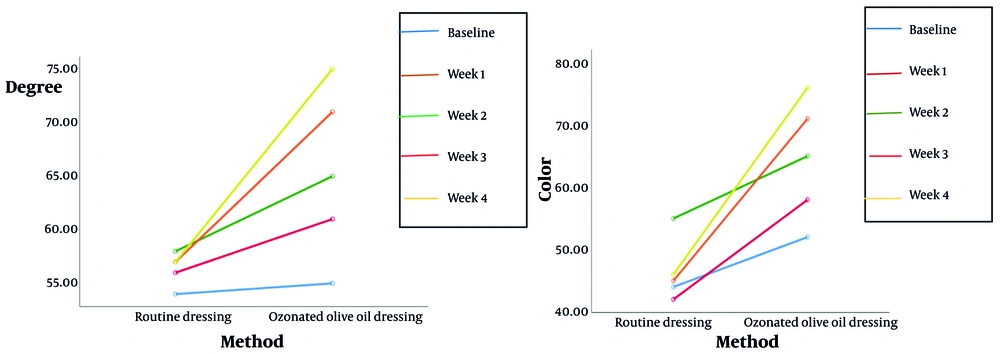
By the end of the study, the intervention group demonstrated significantly better outcomes in ulcer healing. Complete healing occurred in 5.6% of the intervention group compared to 0% in the control group (P = 0.00). Partial healing was more common in the intervention group (91.7% vs. 31.4%), while lack of healing and deterioration were more frequent in the control group (54.3% and 14.3%, respectively; Table 2). Wound grade and surrounding tissue condition improved significantly more in the intervention group by week 4 (P < 0.001). Additionally, wound drainage improved substantially in the intervention group over time, while remaining stable in the control group (P < 0.001; Tables 2 and 3; Figures 2 and 3).
| Variables and Groups | Mean ± SD | P-Value |
|---|---|---|
| Surrounding tissues-baseline | < 0.001 | |
| Control | 46.714 ± 13.823 | |
| Intervention | 44.722 ± 6.755 | |
| Surrounding tissues-week 1 | ||
| Control | 47.428 ± 12.797 | |
| Intervention | 55.694 ± 10.833 | |
| Surrounding tissues-week 2 | ||
| Control | 50.285 ± 14.139 | |
| Intervention | 61.944 ± 10.438 | |
| Surrounding tissues-week 3 | ||
| Control | 49.142 ± 14.425 | |
| Intervention | 67.638 ± 7.971 | |
| Surrounding tissues-week 4 | ||
| Control | 49 ± 14.742 | |
| Intervention | 70.55 ± 7.346 | |
| Drainages-baseline | < 0.001 | |
| Control | 44.571 ± 8.520 | |
| Intervention | 41.388 ± 12.224 | |
| Drainages-week 1 | ||
| Control | 44.857 ± 8.53 | |
| Intervention | 53.88 ± 13.369 | |
| Drainages-week 2 | ||
| Control | 45.714 ± 11.952 | |
| Intervention | 61.66 ± 10.823 | |
| Drainages-week 3 | ||
| Control | 44.285 ± 15.771 | |
| Intervention | 72.50 ± 13.389 | |
| Drainages-week 4 | ||
| Control | 44.285 ± 17.368 | |
| Intervention | 78.611 ± 14.765 | |
| Drainages-baseline | < 0.001 | |
| Control | 190.914 ± 32.191 | |
| Intervention | 192.083 ± 33.792 | |
| Drainages-week 1 | ||
| Control | 191.857 ± 30.966 | |
| Intervention | 229.722 ± 36.799 | |
| Drainages-week 2 | ||
| Control | 198.428 ± 46.696 | |
| Intervention | 253.888 ± 33.702 | |
| Drainages-week 3 | ||
| Control | 196.285 ± 43.102 | |
| Intervention | 282.36 ± 33.348 | |
| Drainages-week 4 | ||
| Control | 196.857 ± 47.249 | |
| Intervention | 384.166 ± 50.66 |
| Variables and Groups | Mean ± SD | P-Value |
|---|---|---|
| Degree-baseline | < 0.001 | |
| Control | 54.8571 ± 13.42110 | |
| Intervention | 55.6944 ± 8.87636 | |
| Degree-week 1 | ||
| Control | 56.4286 ± 10.47205 | |
| Intervention | 61.5278 ± 9.84301 | |
| Degree-week 2 | ||
| Control | 58.1429 ± 11.31668 | |
| Intervention | 65 ± 9.18073 | |
| Degree-week 3 | ||
| Control | 57.1429 ± 12.26459 | |
| Intervention | 71.25 ± 9.66400 | |
| Degree-week 4 | ||
| Control | 57.1429 ± 12.90723 | |
| Intervention | 75.1389 ± 8.74121 | |
| Color-baseline | < 0.001 | |
| Control | 44.428 ± 11.425 | |
| Intervention | 52.77 ± 8.489 | |
| Color-week 1 | ||
| Control | 42.171 ± 11.746 | |
| Intervention | 58.888 ± 8.203 | |
| Color-week 2 | ||
| Control | 55.142 ± 43.74 | |
| Intervention | 65.277 ± 8.77 | |
| Color-week 3 | ||
| Control | 46 ± 10.901 | |
| Intervention | 71.11 ± 8.203 | |
| Color-week 4 | ||
| Control | 46.714 ± 10.77 | |
| Intervention | 76.388 ± 7.983 |
Adverse effects, including sensitivity, bleeding, and infection, were generally low and did not differ significantly between groups (P > 0.05). However, the control group reported slightly more adverse effects overall. Quantitative wound measures, including dimensions and duration of wound formation, showed no significant differences between groups at baseline or during the study (P > 0.05).
Effect size analysis revealed a much stronger impact of the intervention over time compared to the control. By the fourth week, the intervention group demonstrated consistently higher effect sizes, indicating significant improvements in wound outcomes (Table 4).
| Methods and Total | Effect Size | 95% Confidence Interval | |
|---|---|---|---|
| Lower Bound | Upper Bound | ||
| Control | 0.02 | ||
| Baseline | 179.856 | 201.973 | |
| Week 1 | 181.220 | 202.495 | |
| Week 2 | 182.388 | 214.469 | |
| Week 3 | 181.479 | 211.092 | |
| Week 4 | 180.627 | 213.088 | |
| Intervention | 0.878 | ||
| Baseline | 180.649 | 203.517 | |
| Week 1 | 217.271 | 242.173 | |
| Week 2 | 242.486 | 265.292 | |
| Week 3 | 271.078 | 293.645 | |
| Week 4 | 289.754 | 311.912 | |
5. Discussion
The study revealed that while demographic characteristics and wound location were comparable between the intervention and control groups, significant differences emerged in wound severity and healing outcomes. The intervention group showed a higher prevalence of Wagner grade 1 wounds and significantly better wound healing, with a greater percentage of partial improvements compared to the control group.
The results of this study demonstrated that ozonated olive oil significantly improved wound healing, as evidenced by reductions in wound severity, discoloration, surrounding tissue damage, and drainage. Statistical analysis further revealed a substantial effect size of ozonated olive oil compared to standard wound dressings for DFUs. The antimicrobial properties of ozone, coupled with the emollient effects of olive oil, likely contribute to these beneficial outcomes. Ozone's ability to combat biofilm formation and reduce bacterial load may have directly influenced the reduction in wound drainage. Additionally, the anti-inflammatory effects of ozone and olive oil may have mitigated tissue damage and promoted a more favorable healing environment. These findings align with previous studies highlighting the potential of ozone therapy in wound care and further support the exploration of ozonated olive oil as a promising adjunct to standard wound care practices (13).
Our findings align with those of Martinez-Sanchez et al. (as cited by Zhang et al.), who reported a significant reduction in lesion size and perimeter in DFUs following local and rectal ozone insufflation, without any adverse effects. Similarly, Zhang et al. proposed a potential mechanism of action for non-invasive oxygen-ozone therapy, suggesting that it may accelerate DFU healing by upregulating growth factors such as vascular endothelial growth factor, transforming growth factor-β, and platelet-derived growth factor in the early stages of treatment (25).
A placebo-controlled clinical trial by Wainstein et al. further supports the efficacy of ozone therapy, demonstrating significantly higher rates of complete wound closure in patients receiving combined conventional and ozone-oxygen treatment compared to those receiving sham treatment (26). QIN evaluated the clinical efficacy of ozone baths in controlling DFU infections and promoting wound healing, finding that the experimental group exhibited superior outcomes in terms of ulcer reduction compared to the control group (27). Moreover, another research reported improved recovery of DFUs in patients who received a combination of negative pressure wound therapy (NPWT) using vacuum-assisted closure (VAC) and ozone water compared to VAC alone (28).
Despite the diversity of ozone therapy modalities employed in these studies, a consensus has emerged regarding the significant role of this intervention in controlling diabetic wound dimensions.
Based on Table 3, the highest frequency of wound location in the control group is on the heel (45.7%), while the lowest frequency is 8.6%. In the intervention group, 55.6% of wounds are located on the heel, with the lowest frequency of wounds on the foot being 2.8%. The chi-square test shows no significant difference in wound location between the intervention and control groups. These findings align with other research, which also observed a predominance of heel ulcers in diabetic foot studies (29). However, studies have reported significant associations between wound location and healing outcomes, suggesting that further exploration may be warranted (30).
At the end of the study, 91.7% of the intervention group showed partial improvement in wound healing, compared to 54.3% of non-healing cases in the control group. Fisher's exact test revealed a significant difference in wound healing status between groups. The findings corroborate the results of another researcher, who demonstrated that similar interventions improved healing rates significantly (31). However, another study found varying efficacy, highlighting the need for standardizing intervention protocols (32).
Sensitivity was the most common adverse effect in both groups, reported by 65.7% of the control group and all participants in the intervention group. Bleeding was reported in 8.6% of the control group. Fisher's exact test indicated no significant difference in adverse effects between groups. This aligns with other research, which observed minimal adverse effects in similar interventions (33). However, another study reported slightly higher incidences of infection, which may reflect differences in patient demographics or intervention techniques (34).
Wound onset duration is a critical parameter in assessing the healing process and the effects of various interventions on wound recovery. In our study, the mean duration of wound onset was 3 weeks for the control group and two weeks for the intervention group. This indicates a difference in the timing of wound onset between the two groups. However, the independent t-test revealed that this difference was not statistically significant. The impact of wound onset duration on healing outcomes has been the subject of various studies.
This study, while promising, has several limitations. Firstly, the dearth of existing research on ozonated olive oil precluded robust comparisons with previous studies. Secondly, the diverse applications of ozone therapy for DFUs necessitate further investigation to identify the optimal approach. Moreover, the relatively short four-week follow-up period limited the assessment of long-term effects and other potential benefits, such as anti-inflammatory and antimicrobial properties. Despite the limitations of this pilot study, the findings suggest that ozonated olive oil may serve as a valuable adjunct to standard care for DFUs. However, larger-scale, randomized controlled trials are necessary to confirm these findings and to thoroughly explore the potential of this novel therapeutic approach.
5.1. Conclusions
This study provides preliminary evidence supporting the use of ozonated olive oil in the healing of DFUs. Although baseline characteristics did not differ significantly between the groups, the positive outcomes observed with the ozonated treatment indicate a potential benefit. Future research is crucial to confirm these findings and elucidate the precise effects of ozonated olive oil on wound healing. Further investigation is warranted to confirm these findings and explore the mechanisms by which ozonated olive oil may aid in wound healing. Larger-scale studies with diverse populations and extended follow-up periods are needed to validate these results and determine the generalizability of the treatment. Such research will facilitate the integration of ozonated treatments into clinical practice, providing new options for managing DFUs.