1. Background
Chronic kidney disease (CKD) is associated with various cutaneous manifestations including pruritus, xerosis, pigmentation, yellow skin, perforating disorders, calciphylaxis, benign nodular calcification, porphyria cutanea tarda, pseudoporphyria, and nephrogenic systemic fibrosis (1-3). The most common manifestation, xerosis affects 66% to 72% (1, 2), while pruritus affects 24% to 43% patients (1, 2, 4). Pruritus and xerosis often co-exist. The prevalence is higher with more severe CKD and in patients on regular dialysis (1, 2).
The aetiology of uraemic pruritus is multifactorial. Xerosis is a common association (5). Parathormone, histamine, tryptase, uraemic toxins, upregulation of Th1, and interleukin-6 are thought to cause neuropathy and inflammation within the skin that lead to pruritus (6, 7). Central nervous system opiod imbalance is another aetiological factor recently targeted in the management of uraemic pruritus (7). Uraemic pruritus causes multiple morbidities including excoriations and ulcerations that predispose to infection, nodular prurigo, and lichenification (6). Moderate to severe pruritus leads to feeling drained, poor sleep quality, depression, and higher risk of mortality (8).
Local cutaneous changes along with systemic responses contribute to the clinical manifestations in patients with CKD. The interactions between multiple factors in the pathophysiology of both xerosis and pruritus are still unclear. The effect of patients’ fluid volume status on xerosis and pruritus has been poorly investigated. Excessive accumulation of fluid in the interstitial spaces may cause proprioceptive nerve fiber dysfunction due to edema. Is xerosis a marker for increased cutaneous insensible water loss? An objective description of cutaneous biophysical parameters and total body water in CKD patients will contribute to further understand the pathophysiology of pruritus.
2. Objectives
The objectives of this study were to determine the biophysical parameters (transepidermal water loss, hydration, and pH) of the skin in different stages of kidney impairment. We examined the relationship between these cutaneous parameters with biochemical and haematological parameters, severity of uraemic pruritus, and body water content.
3. Methods
A cross sectional study involving adult patients with CKD attending the Nephrology Clinic at a University Medical Center was performed. Inclusion criteria were all patients aged ≥ 50 years old diagnosed with CKD irrespective of stage and primary disease. Exclusion criteria were patients with other skin conditions e.g. eczema and psoriasis and patients on dialysis (peritoneal dialysis or haemodialysis). Informed consent was obtained. Data was collected by a face to face interview and reviewing the patients’ medical records. Blood and urinalysis results in the last four months prior to recruitment were recorded. Haematological and biochemistry parameters included were: haemoglobin, urea, creatinine, calcium, phosphate, fasting blood sugar, HBA1C, alanine transferase, alkaline phosphatase, total cholesterol, triglycerides, low-density lipoproteins (LDL) and high-density lipoproteins (HDL), and urine protein creatinine index (PCI). Estimation of glomerular filtration rate (eGFR) was calculated using chronic kidney disease epidemiology collaboration (CKD-EPI) and the modification of diet in renal disease (MDRD) study equation (9, 10). Symptom of itch was determined by asking the patient to rate a visual analogue score of 0 to 10. Zero means no itch at all while 10 means the most severe itch ever experienced. Transepidermal water loss (TEWL) was measured using Tewameter® TM300. Skin hydration was measured using DermaLab Combo while skin surface pH was measured using Hanna Instruments H199181. Body water content was determined using InBody S10 analyzer. All measurements were performed at eight predetermined sites: anterior aspect of mid forearm bilaterally, anterior aspect of mid-thigh bilaterally, anterior aspect of mid leg (shin) bilaterally, left and right side of the abdomen about four fingerbreadth lateral to the umbilicus.
Sample size calculation was performed using the Power and Sample Size Calculation software (11) based on the results of a previous study that examined TEWL in haemodialysis patients (12). The result was normally distributed with a standard deviation of four, and the difference in mean TEWL of dialysis patients and control was 2.6 (12). The calculated sample size was 90, considering a 20% drop out rate to be able to reject the null hypothesis that the population means of the experimental and control groups are equal with probability (power) of 0.8. The type I error probability was 0.05. Statistical analyses were performed with IBM®SPSS statistical software. Majority of continuous variables were normally distributed; thus, results are expressed in means with standard deviation and parametric tests were used. CKD severity was grouped into mild (stages 1 and 2), moderate (stage 3) and severe (stages 4 and 5). One way ANOVA tested differences in biophysical parameters between the CKD severity groups. Pearson correlation test was used to determine correlations between continuous variables. Multinomial logistic regression was performed to determine independent predictors for severity of pruritus. Age, gender, ethnicity, body mass index, duration of CKD, CKD severity, cutaneous biophysical parameters, haematological, and biochemical parameters were included in the regression analyses. Ethical approval was obtained from the Research Ethics Committee, the National University of Malaysia project code FF-2019-192.
4. Results
A total of 83 patients participated in the study. The mean age was 66.8 ± 8.4 years. There were 42 (50.6%) males and 41 (49.4%) females. The majority was of the Malay ethnicity 47(56.6%), followed by Chinese 30 (36.1%), Indian 5 (6.0%) and 1 (1.2%) from another ethnicity. The mean duration of CKD was 9.33 ± 7.6 years. The aetiology of CKD was diabetes mellitus in 44 (53.0%), hypertension 6 (7.2%), obstructive uropathy 2 (2.4%), and others including autoimmune diseases 31 (37.3%). There were 7 (8.4%) patients with CKD stage 1, another 7 (8.4%) stage 2, 26 (31.3%) stage 3, 34 (41.0%) stage 4, and 9 (10.8%) stage 5. Comorbidities included hypertension 78 (94%), diabetes 59 (71.1%), dyslipidaemia 73 (88.0%), ischaemic heart disease 24 (28.9%), and cerebrovascular disease 5 (6%). Pruritus was present in 22 (26.5%) of the patients, 6 (7.2%) graded their pruritus as mild, 15 (18.1%) moderate, and 1 (1.2%) severe. Characteristics of the study population are summarized in Table 1.
| Parameters | Values |
|---|---|
| Age, y | 66.77 ± 8.421 |
| Gender | |
| Male | 42 (50.6) |
| Female | 41 (49.4) |
| Ethnicity | |
| Malay | 47 (56.6) |
| Chinese | 30 (36.1) |
| Indian | 5 (6.0) |
| Others | 1 (1.2) |
| BMI | |
| Underweight | 5 (6.0) |
| Normal | 27 (32.5) |
| Overweight | 23 (27.7) |
| Obese | 28 (33.7) |
| Duration of CKD | 9.33 ± 7.56 |
| Aetiology of CKD | |
| Type II diabetes mellitus | 44 (53) |
| Hypertension | 6 (7.2) |
| Others | 31 (37.3) |
| Obstructive uropathy | 2 (2.4) |
| CKD severity | |
| Stage 1 | 7 (8.4) |
| Stage 2 | 7 (8.4) |
| Stage 3 | 26 (31.3) |
| Stage 4 | 34 (41) |
| Stage 5 | 9 (10.8) |
| Co morbidities | |
| Hypertension | 78 (94) |
| Diabetes | 59 (71.1) |
| Dyslipidaemia | 73 (88) |
| Ischemic heart disease | 24 (28.9) |
| Cerebrovascular disease | 5 (6) |
| Pruritus | |
| Yes | 22 (26.5) |
| No | 61 (73.5) |
| Pruritus score (0 - 10) | |
| Asymptomatic | 61 (73.5) |
| Mild (1 - 3) | 6 (7.2) |
| Moderate (4 - 7) | 15 (18.1) |
| Severe (8 - 10) | 1 (1.2) |
aValues are expressed as mean ± SD or No. (%).
The results of skin biophysical parameters and body water are presented in Table 2. TEWL measured at the forearm, thigh, shin, abdomen, and the mean of all these sites were not significantly different between mild, moderate, and severe CKD. Skin surface pH decreased from mild to moderate CKD (except at the abdomen), followed by an increased in severe CKD. However, the differences were not significant. The values of skin hydration showed an increase from mild to moderate CKD at the forearm and abdomen while the values in severe CKD were consistently lower than mild CKD at all sites. The differences in skin hydration were not significant. Water content at the forearm was significantly higher with more severe CKD (Table 2). The total body water and water contents at other sites were not significantly different.
| Stage 1 and 2 (N = 14) | Stage 3 (N = 26) | Stage 4 and 5 (N = 43) | P Value | |
|---|---|---|---|---|
| TEWL | ||||
| Forearm | 9.73 ± 2.11 | 10.42 ± 3.13 | 9.71 ± 1.64 | 0.43 |
| Mid-thigh | 10.23 ± 4.09 | 9.95 ± 2.70 | 9.18 ± 1.39 | 0.26 |
| Shin | 9.23 ± 1.49 | 9.53 ± 1.71 | 9.80 ± 1.28 | 0.42 |
| Abdomen | 9.62 ± 5.60 | 7.77 ± 2.74 | 7.98 ± 2.62 | 0.21 |
| All sites | 9.71 ± 2.69 | 9.42 ± 1.93 | 9.17 ± 0.93 | 0.56 |
| pH | ||||
| Forearm | 5.07 ± 0.54 | 4.99 ± 0.52 | 5.30 ± 0.58 | 0.08 |
| Mid-thigh | 5.23 ± 0.66 | 5.06 ± 0.58 | 5.30 ± 0.55 | 0.25 |
| Shin | 5.22 ± 0.63 | 5.04 ± 0.61 | 5.28 ± 0.57 | 0.28 |
| Abdomen | 5.38 ± 0.45 | 5.47 ± 0.60 | 5.67 ± 0.58 | 0.16 |
| All sites | 5.22 ± 0.50 | 5.14 ± 0.53 | 5.39 ± 0.50 | 0.15 |
| Hydration | ||||
| Forearm | 216.68 ± 56.72 | 221.88 ± 87.07 | 183.20 ± 58.21 | 0.05 |
| Mid-thigh | 140.50 ± 42.34 | 135.06 ± 78.19 | 115.45 ± 32.50 | 0.17 |
| Shin | 122.79 ± 39.06 | 100.10 ± 38.35 | 101.90 ± 36.08 | 0.15 |
| Abdomen | 170.11 ± 50.76 | 183.73 ± 12.53 | 164.20 ± 6.93 | 0.33 |
| All sites | 162.52 ± 30.42 | 160.19 ± 54.39 | 141.19 ± 34.49 | 0.10 |
| Body water | ||||
| Arm | 0.38 ± 0.01 | 0.38 ± 0.01 | 0.38 ± 0.01 | 0.00 |
| Trunk | 0.39 ± 0.01 | 0.38 ± 0.02 | 0.39 ± 0.02 | 0.19 |
| Leg | 0.38 ± 0.05 | 0.39 ± 0.03 | 0.40 ± 0.02 | 0.14 |
| Total | 0.39 ± 0.01 | 0.38 ± 0.03 | 0.39 ± 0.02 | 0.22 |
aValues are expressed as mean ± SD.
Table 3 shows the relationship between cutaneous biophysical parameters and total body water content with pruritus. Severity of pruritus was grouped into none, mild, and moderate plus severe. Only one patient graded his pruritus as severe. There were no significant differences in the values of TEWL, hydration, pH, and total body water with pruritus severity. Correlation analyses between pruritus score with cutaneous biophysical parameters, haematological, and biochemical parameters were non-significant.
| Pruritus | ||||
|---|---|---|---|---|
| None (N = 61) | Mild (N = 6) | Moderate and Severe (N = 16) | P Value | |
| TEWL | 9.22 | 9.38 | 9.75 | 0.53 |
| pH | 5.24 | 5.67 | 5.29 | 0.14 |
| Hydration | 156.12 | 126.64 | 139.24 | 0.12 |
| Total body water | 0.388 | 0.37 | 0.40 | 0.06 |
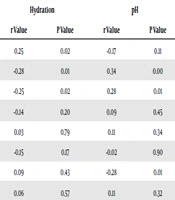
There was no significant correlation between TEWL with eGFR, urea, creatinine, calcium, phosphate, haemoglobin, and urine PCI. There was a positive, significant correlation between hydration and eGFR with r = 0.25 and P = 0.02. Hydration correlated negatively with urea, r = -0.28, P = 0.01. Significant negative correlation between hydration with creatinine was observe with r = -0.25, P = 0.02. Surface pH correlated with urea with r = 0.34, P = 0.00, creatinine r = 0.28, P = 0.01, and haemoglobin r = -0.28, and P = 0.01 (Table 4). Total body water showed a negative significant correlation with TEWL, r = -0.25, P = 0.02 but not with hydration, r = 0.06, P = 0.57 or pH, r = 0.11, P = 0.32. There was no significant result from multinomial logistic regression to determine independent predictors for severity of pruritus.
| Parameters | TEWL | Hydration | pH | |||
|---|---|---|---|---|---|---|
| r Value | P Value | r Value | P Value | r Value | P Value | |
| eGFR | 0.04 | 0.72 | 0.25 | 0.02 | -0.17 | 0.11 |
| Urea | -0.14 | 0.20 | -0.28 | 0.01 | 0.34 | 0.00 |
| Creatinine | -0.07 | 0.51 | -0.25 | 0.02 | 0.28 | 0.01 |
| Calcium | 0.05 | 0.69 | -0.14 | 0.20 | 0.09 | 0.45 |
| Phosphate | -0.18 | 0.11 | 0.03 | 0.79 | 0.11 | 0.34 |
| Urine PCI | -0.06 | 0.62 | -0.15 | 0.17 | -0.02 | 0.90 |
| Haemoglobin | 0.07 | 0.55 | 0.09 | 0.43 | -0.28 | 0.01 |
| Total body water | -0.25 | 0.02 | 0.06 | 0.57 | 0.11 | 0.32 |
5. Discussion
Cutaneous and systemic factors contribute to development of cutaneous manifestations of CKD. Changes within the epidermis, which affect the skin’s barrier function, play a role, especially in development of pruritus and xerosis. Both symptom and sign are essential features of endogenous eczema where disruption of the epidermal barrier is a fundamental aspect in its pathophysiology. Impairment of stratum corneum integrity reflected by increased TEWL (13), higher skin surface pH (13, 14), reduced glycerol content (13), and lower hydration (13-15) has been demonstrated in case control studies involving CKD patients on dialysis. One study did not demonstrate significant differences in TEWL (12). Skin hydration was lower in peritoneal dialysis patients compared to controls, however, there were no differences in the skin hydration of patients who were on haemodialysis (16). All case control studies were performed on patients who were on dialysis (12-16).
Changes in TEWL, hydration, and pH are expected to worsen with increased severity of CKD in non-dialysis patients based on the findings of these case control studies (12-16). However, these were not demonstrated in our cohort of patients with CKD stage 1 - 5. A trend towards increased pH and reduced hydration was observed, however, there were no significant differences in the values of pH and hydration at multiple sites. The trend in TEWL values differed according to site, the mean of all sites showed non-significant reduction in TEWL with more severe CKD. Xerosis and pruritus commonly affect the extremities (1), we measured all biophysical parameters at both upper and lower limbs but did not see significant differences. Results from correlation analyses suggested impairment in hydration and pH with CKD severity. Higher total body water was associated with impaired TEWL. TEWL, hydration, and pH are affected by age and gender (17-19). TEWL is higher in females until the age of around 50 years. Skin pH is higher in females and with older age. Hydration increases to a peak at 30 to 40 years and then declines with older age (17-19). Our study included patients aged ≥ 50 years to minimize the effect of age and there were equal number of males and females. Epidermal barrier dysfunction most likely worsens with chronicity of severe CKD as seen in dialysis patients rather than related to severity of CKD. Alternatively, the changes in the epidermal barrier could be enhanced by dialysis. Rapid and frequent dynamic changes in total body water during maintenance haemodialysis could contribute to the state of water loss of the skin.
Our data demonstrated correlations between increasing CKD severity with lower skin hydration and higher skin pH. Reduced skin hydration, increased pH and increased TEWL are well established characteristics of eczematous skin. Xerosis and eczematous skin are common in dialysis patients and patients with CKD (1, 2). To the best of our knowledge, the relationship between total body water with cutaneous biophysical parameters has not been investigated before. In our patients, increased body water was associated with reduced TEWL. Stratum corneum hydration has been demonstrated to improved post-dialysis compared to pre-dialysis but its effect on TEWL was not documented (15). These finding suggest intra and extra-vascular fluid status inversely affect skin hydration and TEWL.
Pruritus is more prevalent in patients who had been on haemodialysis for longer compared to those newly started on haemodialysis and non-dialysis CKD (1, 2, 8). Severity of pruritus in haemodialysis patients is related to the male gender, co-existing lung disease, heart failure, neurological disease, hepatitis C, liver disease with ascites, higher serum calcium or phosphorus, lower serum albumin, and increased white blood cell count (8). More recent developments on CKD related pruritus focused on peripheral neuropathy and upregulation of central µ-opioid receptors (6, 20). However, use of emollients to relieve pruritus and xerosis has been shown to be beneficial and is recommended in the management of uremic pruritus with or without xerosis (5, 6, 20). The frequency of pruritus is higher when xerosis is present (1, 5). Xerosis maybe one of the stimuli that drive altered responses from cutaneous nerve endings resulting in itch. The most likely reason we did not find any relationship between pruritus with epidermal biophysical parameters between CKD stages was due to the small number of patients with xerosis. We did not properly document the presence of xerosis, which is a limitation in this study. However, clinically apparent xerosis was reflected objectively by biophysical measurements.
5.1. Conclusions
There were no differences in cutaneous biophysical parameters in non-dialysis CKD stage 1 - 5 patients. Eczematous changes of the skin occur with increasing severity of CKD as demonstrated by correlations between higher urea and creatinine with lower hydration and higher pH. However, we did not observe an increase in TEWL values, which is an essential feature of eczematous skin. Pruritus was not related to CKD stage, cutaneous biophysical, haematological, and biochemical parameters. We found increased total body water associated with lower TEWL, which suggested that insensible water loss through cutaneous evaporation was not influenced by patients’ volume status. Our results need confirmation by further studies involving larger number of patients in each CKD stage. We recommend routine skin examination in moderate to severe CKD patients and regular use of moisturizer should be advised if these changes are observed.