1. Introduction
Pityriasis rubra pilaris (PRP) is a group of chronic papulosquamous disorders manifesting hyperkeratotic follicular papules and forming salmon-colored plaques with white scales and commonly accompanied by palmoplantar keratoderma (1, 2). The peak incidence of PRP is in early childhood (0-10-years-old), late childhood (11-19-years-old), and adulthood (40-60-years-old) with an approximate prevalence of 1:5,000 to 1:50,000 (3, 4). Children are less affected than adults and approximately include 40% of all PRP cases. In children, PRP is more common in boys than in girls with a ratio of 3:2 (5).
The PRP treatment remains challenging, especially for some cases unresponsive to topical or systemic treatment. The paucity of the case makes it difficult to do controlled clinical trials to evaluate the efficacy and safety of the treatment. Published controlled clinical trials in PRP are not available up to now; therefore, the treatment of PRP is commonly based on case reports and case series (5, 6). Topical calcipotriol is used as the first-line topical therapy even though there is no study to prove the effectiveness (1).
2. Case Presentation
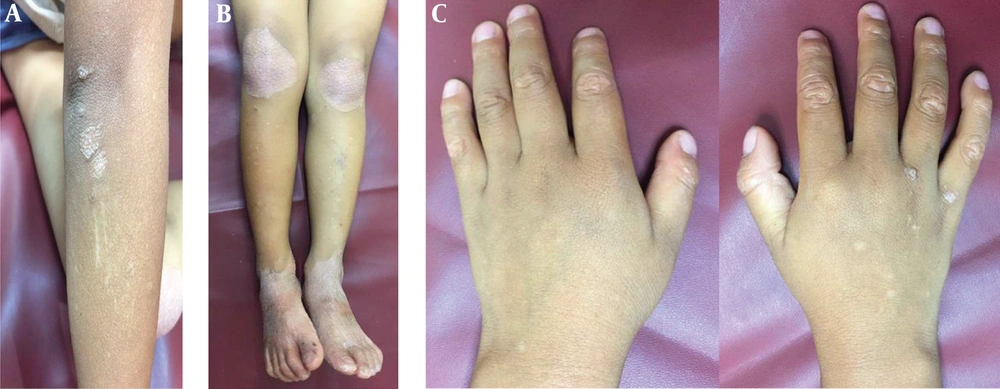
A four-year-old boy presented with a chief complaint of scaly red lesions on his elbows, knees, and back associated with gradual thickening palms and soles since he was 3.5-months-old, with no history of trauma. We noticed the appearance of papules at his fingers and back of his hands. The papule and plaques appeared simultaneously and were not progressive. No family member had the same complaint. The patient used baby soap for daily cleansing and coconut oil as a daily moisturizer. He had been treated with 2.5% hydrocortisone cream for about one year without any improvement.
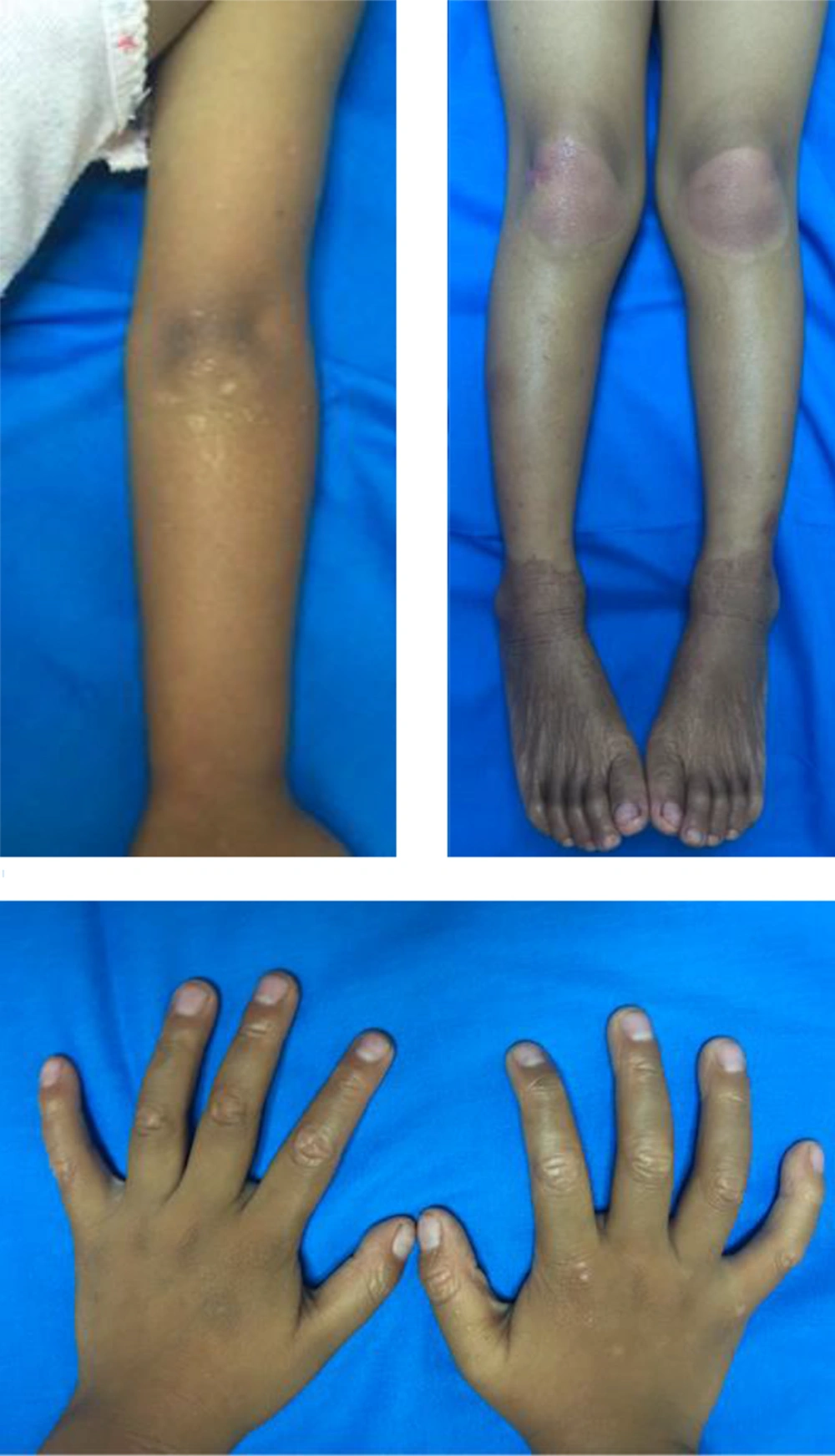
The general physical examination was within normal limits. In a dermatologic examination, we found erythematous plaques on his elbows and knees, hyperkeratotic papules at his fingers with nutmeg grater signs (Figure 1), and palmoplantar keratoderma with a waxy appearance. The severity was determined by the Psoriasis area and severity index (PASI), as there was no guideline to monitor the disease severity in PRP; the patient got a PASI score of 3. The dermoscopic examination of lesions showed white scales in salmon-colored background and hairs in the center (Figure 2).
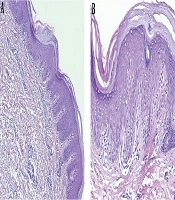
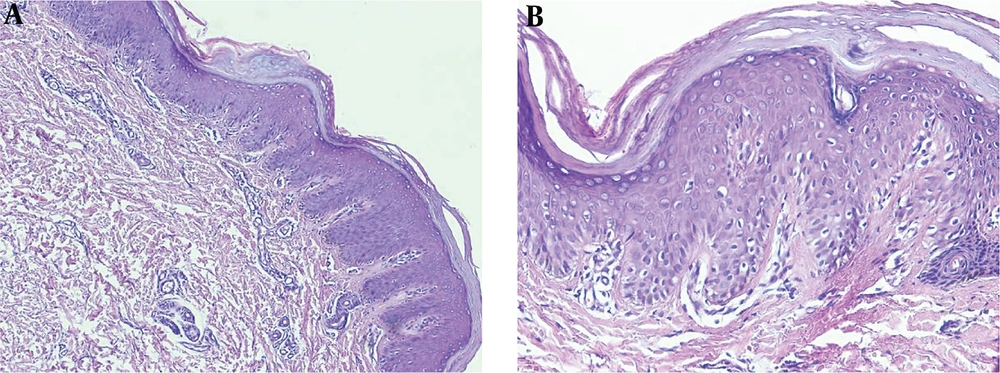
Routine blood examination, thyroid function, and C-reactive protein were normal and ASO and rheumatoid factor were negative. The histopathologic examination revealed alternating parakeratosis and orthokeratosis in vertical and horizontal directions (checkerboard pattern) suitable for PRP histopathologic findings; hyperplasia psoriasiform and suprapapillary plate thickening were also found in this case. The dermis was filled with fibrocollagenous tissue, lymphocytic infiltrates in the vessels, hair follicles, and eccrine glands (Figure 3).
The patient was diagnosed with circumscribed juvenile PRP (type IV) and treated with 0.005% calcipotriol ointment every 12 h and an oral antihistamine. After 10 weeks, lesions were clinically improved (Figure 4) and pruritus reduced. The PASI score decreased to 0.4. The patient is currently using calcipotriol ointment but oral antihistamines have stopped.
3. Discussion
Pytiriasis rubra pilaris is a group of papulosquamous disorders characterized by chronic inflammation that manifests as hyperkeratotic follicular papules, forming salmon color plaque topped with white scales, and often accompanied with palmoplantar keratoderma (1, 2). Griffiths classified PRP into six types based on clinical characteristics: type I (classic adult), type II (atypical adult), type III (classic juvenile), type IV (circumscribed juvenile), type V (atypical juvenile), and type VI (human immunodeficiency virus-associated) (1). Circumscribed juvenile PRP (type IV) is the most common type of PRP in children, accounting for 25% of all PRP patients. The onset occurs commonly at pre-pubertal age (3-10-years-old); however, it may manifest as early as less than one-year-old (5, 7). This type is characterized by well-demarcated scaly and erythematous plaques on the elbows and knees; these lesions do not progress to widespread types I and III (1), similar to our patient in this case.
The etiology of PRP is still unknown, even though it has been found since the early 19th century (1, 8). There are several theories associated with this condition, such as vitamin A deficiency, low-level retinol-binding protein (RBP), autoimmune diseases, traumas, genetic factors, infections, and carcinomas. In most cases, the triggering factors of PRP are unknown (9, 10).
Genetic factors with an autosomal dominant of inheritance also play a role in PRP (1). Family history of PRP is found in 6.5% of the cases and most of the familial cases belong to type V (atypical juvenile) (11). A genetic factor that can cause PRP is the mutation in CARD14, which is often found in the majority of patients with PRP; the NK-kB signal activator has also a role in skin inflammation (8). Our patient had the characteristic signs of circumscribed juvenile PRP, i.e. salmon color scaly papules and plaques with well-defined borders on the elbows and knees; palmoplantar keratoderma, and follicular keratotic papules in the back of fingers and feet, associated with pruritus. The initial appearance of lesions was located on the back of hands and feet, progressing to knees, elbows, and fingers. We need further research to prove whether the patient in our case has a mutation of CARD14.
Psoriasis is the major entity for PRP differential diagnosis. The presence of palmoplantar keratoderma, keratotic follicular papules, the classic island of sparing of the trunk, a finer scale, and family history of psoriasis help differentiate PRP from psoriasis (5). In PRP, dermoscopy shows yellow and white scales and keratotic plugs (black circle) in the yellow-red-black ground, while psoriasis shows white scales in light red background (12). The absence of both neutrophilic migration towards the epidermis and Munro’s microabscesses in histopathologic findings can help differentiate PRP from psoriasis (5).
The characteristic histopathologic findings of PRP are alternating parakeratosis and orthokeratosis horizontally and vertically, also known as the checkerboard pattern. Psoriasiform hyperplasia, suprapapillary plate thickening, keratinous plug in the infundibular follicle, and perivascular lymphocytic infiltrate are commonly found (1, 13). The paucity of the case makes it difficult to do controlled clinical trials to evaluate the effectiveness and safety of the treatment. Previous publications regarding PRP treatment were only case reports or case series (1, 8). Generally, the treatment of PRP involves topical, systemic, or physical treatment; the combination of these treatments is also warranted (1).
First-line therapy of PRP includes emollients, keratolytics, and vitamin D3 for topical treatment, while systemic treatment includes retinoids, methotrexate, and triple antiretroviral (for HIV-associated type). Other physical modalities such as photochemotherapy, phototherapy, and extracorporeal photopheresis also can be used for PRP treatment (1). Calcipotriol is a synthetic vitamin D3 analog (calcitriol) that binds to vitamin D3 receptor in keratinocytes. Vitamin D inhibits keratinocyte proliferation, epidermal differentiation, inflammation by inhibition of IL-2 and IL-6, IFN-gamma transcription, granulocyte-macrophage colony-stimulating factor (GM-CSF) messenger mRNA, cytotoxic T cell, NK cell, and the release of arachidonic acid from neutrophils. The affinity of calcipotriol is similar to that of calcitriol, but the risk of hypercalcemia is lower since calcipotriol is metabolized extensively in topical use. Calcipotriol has a 100 - 200 times lower risk of hypercalcemia than calcitriol. The maximum dose of calcitriol ointment for children younger than six-years-old is 30 grams/week (14, 15).
Our patient was treated with 0.005% calcipotriol ointment every 12 h. The monitoring of treatment noted a reduction of 90% in the PASI score after 10 weeks. The PASI score was used to evaluate the treatment outcome because no PRP score was available yet. Our patient had a normal calcium level with no side effects after calcipotriol use.
Pityriasis rubra pilaris is a self-limiting condition with expected spontaneous remission within two years. However, spontaneous remission is less likely to occur in circumscribed juvenile PRP (type IV) than in classical PRP (type I or III), occurring in 32% of the patients. Lesions in circumscribed juvenile PRP could be settled for more than three years in 67% of the patients (5, 8). Our patient had lesions for more than three years without spontaneous remission.
Prognosis is not related to an acute or gradual onset of PRP. The recurrence was found in 17% of the patients usually in the same area (5, 8). Our patient improved after 10 weeks with no worsening into generalized lesions or erythroderma and without the involvement of other organ systems. Family education is important, as it may recur with varied duration in 17% of cases (9).