1. Introduction
The use of a biologic agent, ustekinumab, for psoriasis has lead to an observation of tumor growth retardation in a patient with unresectable pancreatic cancer. This case report highlights a pre-liminary role of interleukin 23 blockade in altering the tumor microenvironment of certain cancers to influence their growth and metastases which warrants further investigation as a potential target in cancer therapy.
2. Case Presentation
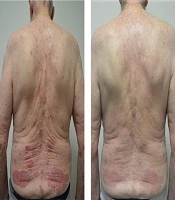
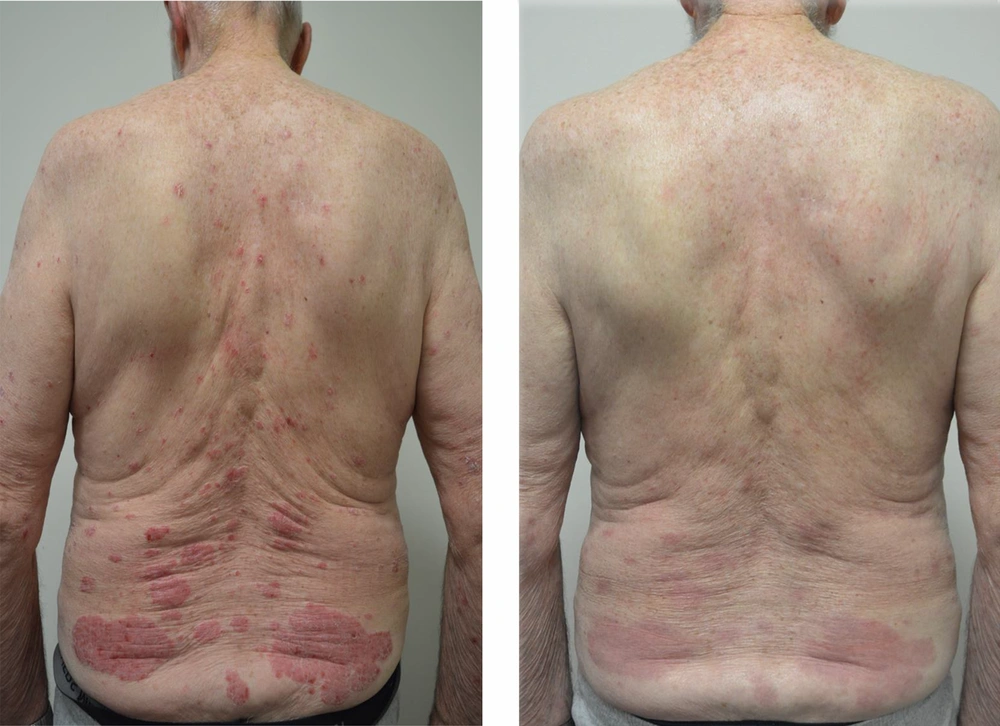
We present a case of a 86 year old male with 40 year history of psoriasis who was commenced on ustekinumab in 2015. Since commencing ustekinumab his baseline PASI reduced from 26 to 2. In December 2017 he was diagnosed with pancreatic adenocarcinoma after being investigated for pancreatitis and obstructive jaundice. A fine needle aspirate and staging CT scan revealed a stage IIB, T1N1M0, locally advanced adenocarcinoma of the head of the pancreas. The patient was considered for a total pancreatectomy however after a multidisciplinary hearing a decision was made towards palliative management with a PEJ tube insertion given the combination of age, comorbidities such as ischaemic heart disease and atrial fibrillation and the size of the tumor. Ustekinumab was temporarily withheld in 2018 from January to May while he was being investigated. Ustekinumab was restarted in May 2018 as his PASI increased to 15.9 (Figure 1). A monitoring CT scan after 3 doses of ustekinumab (45 mg, 45 mg and 90 mg), revealed moderate resolution of the pancreatic lesion from 3 × 8 cm in April 2018 to 2.3 × 5 cm in July 2018 (Table 1). Serial three monthly CT scans since then have revealed stable disease with no metastases and no significant change in the size of the tumor. He remained on 90 mg of ustekinumab every 3 months and the most recent scan in February 2020 has demonstrated no significant increase in tumor size. His other medications include amiodarone, dabigatran, digoxin, pantoprazole and ezetimibe. They remained unchanged since his diagnosis of pancreatic adenocarcinoma.
| 2018 | 2019 | 2020 | |
|---|---|---|---|
| Tumour size on CT imaging, cm | 3 × 8 | 2.3 × 5 | 5.3 × 4.4 |
| Ustekinumab regimen, mg | 45 Q3 monthly | 90 Q3 monthly | Single dose of 45 |
| CA19.9 level, U/L | 23 | 13 | 7 |
3. Discussion
The overall three year survival rate for inoperable stage IIB pancreatic adenocarcinoma from the National Cancer Database is 7.7 per cent and the five year survival is 2 per cent (1). This patient was told his prognosis was 6 weeks however he has survived for more than 29 months in good health with no PEJ tube and no active treatment since the time of diagnosis. Given the prognosis, this finding is interesting and we believe there exists a potential relationship between targeted immunosuppression and tumor activity that is yet to be determined.
To date no clinical trials have been conducted assessing the role of ustekinumab in tumor regression however interleukin 23 (IL-23) has been a focus of interest in clinical oncology.
IL-23 is a pro-inflammatory cytokine believed to serve a critical role in the tumor microenvironment (TME) of several malignancies (2). Tumors such as pancreatic and colorectal cancer are reported to be rich in myeloid cells such as myleoid derived suppressor cells (MDSC) and/or tumor-associated macrophages (TAM) (3). IL-23 is secreted by TAMs and it has been suggested that IL-23 and TGF-β both modulate inflammation in the TME (4). In pancreatic cancer patients, higher serum IL-23 levels were found compared to healthy volunteers (P = 0.015 and P = 0.02) and in particular higher circulating levels of IL-23 and IL-17 were demonstrated in stage III-IV tumors than stage I-II tumors (P = 0.04 and P = 0.036) (5). Scheidt et al, found administration of anti-CD40 and anti-IL-23 monoclonal antibodies interferes with the balance of IL-12 and IL-23 levels, suppressing tumor (mammary carcinoma and lung) metastases (6). Hussain et al. (7) propose that IL-23 mediated tumor progression is context dependent. For example, in duodenal/colon carcinoma IL-23 leads to tumorigenesis through pre-malignant lesions that develop into cancer in an inflammatory mediated manner however IL-23 is not associated with tumorigenesis in head and neck carcinoma (7) These studies implicate an important role of IL-23 in tumor growth and metastases however the directionality in different cancers is yet to be confirmed.
The diagnosis of pancreatic adenocarcinoma after commencing ustekinumab ponders an association between immunosuppression and tumorigenesis. However among 12,000 patients with psoriasis, treatment with methotrexate or ustekinumab was not associated with increased risk of malignancy versus no exposure after 12months or longer (8)