1. Background
Cutaneous biopsies are performed for a variety of reasons, most commonly to assist dermatologists in establishing or verifying a diagnosis. The number of skin biopsies performed in the last decade has increased rapidly (1, 2). It is unclear whether this reflects an increase in incidence of clinically atypical neoplasms, patient awareness and detection, or changes in practice habits.
Academic institutions frequently serve as referral centers for complex and rare entities in dermatopathology, as consultation in this subspecialty is supported by niche expertise, access to obscure immunohistochemical stains and molecular tools, consensus, and additional medicolegal protection. However, analysis of the most common diagnoses and variety of diagnoses encountered in academic dermatopathology overall are lacking in the literature.
Dermatopathology represents a significant portion of dermatology residency training, with specific guidelines for instruction set forth by the Accreditation Council for Graduate Medical Education (ACGME), including board certification for instructors and Milestones® evaluations for learners (3). In clinical dermatology, optimal training is achieved with a combination of didactic and clinical instruction; the latter includes evaluation and management of patients with a wide variety of common and uncommon cutaneous diagnoses. In current models of academic dermatology, high-volume clinics form the basis for clinical instruction. Learners in dermatopathology similarly benefit high-volume caseloads which support both heuristic and analytic diagnosis. However, dermatopathology caseloads in many academic institutions have progressively diminished over the last decade, in part due to a shift in volume to large private and corporate laboratories (4-7).
2. Objectives
It remains unclear how many biopsy specimens residents in dermatology and fellows in dermatopathology should evaluate for sufficient clinical exposure to a wide range of common and rare diagnoses prior to independent practice.
3. Methods
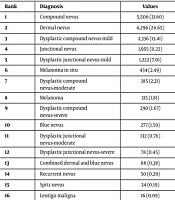
Representing 85,785 specimens, all consecutive dermatopathology reports from the Department of Dermatology at the University of Florida College of Medicine, over a period of 12 months (January 1, 2017 to December 31, 2017, inclusive), were individually reviewed. Diagnoses for non-excisional cases were counted and reported as percentages of the overall caseload and ranked in order of frequency (Table 1). From this data, all melanocytic neoplasms reported were separately stratified and ranked in order of frequency (Table 2).
| Rank | Diagnosis | Values |
|---|---|---|
| 1 | Seborrheic keratosis | 11,079 (14.3) |
| 2 | Basal cell carcinoma | 9,755 (12.6) |
| 3 | Squamous cell carcinoma | 8,175 (10.5) |
| 4 | Actinic keratosis | 7,808 (10.0) |
| 5 | Compound nevus | 5,506 (7.1) |
| 6 | Dermal nevus | 4,296 (5.5) |
| 7 | Wart | 3,621 (4.7) |
| 8 | Solar lentigo | 2,540 (3.3) |
| 9 | Dysplastic compound nevus-mild | 2,336 (3.0) |
| 10 | Epidermal inclusion cyst | 2,138 (2.7) |
| 11 | Prurigo nodularis | 2,018 (2.6) |
| 12 | Junctional nevus | 1,955 (2.5) |
| 13 | Skin tag | 1,380 (1.8) |
| 14 | Lichen planus-like keratosis | 1,366 (1.8) |
| 15 | Dysplastic junctional nevus-mild | 1,222 (1.6) |
| 65,195 (83.0) | ||
| 16 | Dermatofibroma | 853 |
| 17 | Melanoma/in situ melanoma | 749 |
| 18 | Hemangioma/angioma | 600 |
| 19 | Angiofibroma | 530 |
| 20 | Neurofibroma | 485 |
| 21 | Scar | 400 |
| 22 | Dysplastic compound nevus-moderate | 385 |
| 23 | Pilar cyst | 370 |
| 24 | Spongiotic dermatitis-subacute | 360 |
| 25 | Dermal hypersensitivity reaction | 331 |
| 26 | Spongiotic dermatitis-chronic | 294 |
| 27 | Dysplastic compound nevus-severe | 290 |
| 28 | Sebaceous hyperplasia | 278 |
| 29 | Blue nevus | 277 |
| 30 | Nail with no evidence of onychomycosis | 269 |
| 31 | Arthropod bite | 245 |
| 32 | Acute folliculitis | 243 |
| 33 | Eczematous dermatitis-subacute | 240 |
| 34 | Pyogenic granuloma | 214 |
| 35 | Lipoma | 207 |
| 36 | Psoriasis | 203 |
| 37 | Excoriation | 191 |
| 38 | Contact dermatitis-acute | 190 |
| 39 | Chondrodermatitis nodularis helicis | 178 |
| 40 | Onychomycosis | 174 |
| 41 | Psoriasiform dermatitis | 162 |
| 41 | Keratin granuloma | 137 |
| 42 | Dysplastic junctional nevus-moderate | 132 |
| 43 | Granuloma annulare | 128 |
| 44 | Lichen Planus | 120 |
| 45 | Keloid | 108 |
| 74,538 (95.0) |
aValues are expressed as No. (%).
| Rank | Diagnosis | Values |
|---|---|---|
| 1 | Compound nevus | 5,506 (31.60) |
| 2 | Dermal nevus | 4,296 (24.65) |
| 3 | Dysplastic compound nevus-mild | 2,336 (13.41) |
| 4 | Junctional nevus | 1,955 (11.22) |
| 5 | Dysplastic junctional nevus-mild | 1,222 (7.01) |
| 6 | Melanoma in situ | 434 (2.49) |
| 7 | Dysplastic compound nevus-moderate | 385 (2.21) |
| 8 | Melanoma | 315 (1.81) |
| 9 | Dysplastic compound nevus-severe | 290 (1.67) |
| 10 | Blue nevus | 277 (1.59) |
| 11 | Dysplastic junctional nevus-moderate | 132 (0.76) |
| 12 | Dysplastic junctional nevus-severe | 78 (0.45) |
| 13 | Combined dermal and blue nevus | 66 (0.38) |
| 14 | Recurrent nevus | 50 (0.29) |
| 15 | Spitz nevus | 34 (0.19) |
| 16 | Lentigo maligna | 16 (0.09) |
| 17 | Cellular blue nevus | 14 (0.08) |
| 18 | Pigmented spindle cell nevus of Reed | 11 (0.06) |
| 19 | Deep penetrating nevus | 8 (0.04) |
| 17,425 (100.0) |
aValues are expressed as No. (%).
4. Results
In the 2017 calendar year, 56,713 dermatopathology cases were reported by three coauthors of this manuscript. The total number of specimens was 85,785, averaging 1.51 specimens per case. Approximately 14% of these cases represented intradepartmental or institutional referrals. In contrast, 86% of cases were referrals from board-certified dermatologists and mid-level physician extenders employed in a variety of practice settings in the state of Florida: private group or solo practice, corporate practice, and hospital-based practice. Non-excisional biopsy specimens for initial review totaled 77,570 specimens (90.4%). Of all biopsies, 7,432 (8.7%) represented excisional specimens of previously biopsied melanomas, BCCs, cSCCs or dysplastic nevi. The remaining 783 (0.9%) specimens were consultations of previously reviewed biopsies referred for a second opinion. Of these specimens, 92% were melanocytic lesions, 6% represented non melanocytic tumors, and lastly 2% were inflammatory processes.
The 15 most frequently reported diagnoses accounted for 65,195 (84 percent) of all biopsies referred for initial review, and an additional 30 diagnoses (Table 1) accounted for 9,343 (12%) of this subset. The remaining 210 diagnoses accounted for only 2,592 (4%) of this group and reflected 440 neoplastic and 2,192 inflammatory or infectious entities. The three most common diagnoses overall were seborrheic keratosis (14.3%) followed by basal cell carcinoma (12.6%) and squamous cell carcinoma (10.5%). However, if combined into a single diagnostic grouping, benign melanocytic nevi (junctional, compound, and dermal nevi) would represent the most common entity, accounting for 15.1% all specimens. Among the total number of biopsy specimens referred for initial review, 90.7% represented neoplastic diagnoses, and 9.3% represented inflammatory or infectious entities.
Among all tumors, 24.8% were melanocytic, while 75.2% were nonmelanocytic neoplasms. Within melanocytic tumors (Table 2), 765 (4.4%) were malignant, including melanoma and melanoma in situ/lentigo maligna. Dyplastic nevi represented 4,443 (25.5%) of cases, while the remaining 70.1% were benign melanocytic nevi as discussed above. Within nonmelanocytic tumors, 34% were malignant, with a preponderance of basal cell carcinomas and squamous cell carcinomas.
Excisional specimens for previously biopsied dysplastic nevi or malignant tumors accounted for less than 10% of overall specimens. The most frequently encountered excision was for basal cell carcinoma (35.6%), followed by excision of dysplastic nevus (30.3%) and squamous cell carcinoma (27.3%). Excisions of melanoma or melanoma in situ represented only 6.8% of all excisional specimens. The most likely excisional specimen without residual tumor was for dysplastic nevus (87.3%), followed by squamous cell carcinoma (70.3%), melanoma (67.5%), and basal cell carcinoma (53.4%). Among excisional specimens with at least one involved margin, the most frequently encountered residual tumor was melanoma (4.9%). Excisions of basal cell and squamous cell carcinomas had residual tumors with at least one positive margin in 2.9% and 2.5% of cases, respectively. Dysplastic nevi were associated with the lowest frequency of excisional specimens with at least one positive margin (0.9%).
5. Discussion
The number of skin biopsies performed has progressively increased over the last thirty years. Based on the data reflecting Medicare beneficiaries from 1986 to 2001, the number of skin biopsies increased by approximately 10% per year (8). While this trend is ongoing, this rate has slowed slightly in the first decade of this century to approximately 6% per year (1, 2).
Similar to prior studies (9, 10), nevi (including dysplastic nevi), basal cell carcinoma, squamous cell carcinoma and seborrheic keratosis are the four most common diagnoses, although the most commonly reported diagnosis differs. After analyzing 85,785 specimens, our study identified nevi (including dysplastic) as the most common diagnosis among these four entities. Green et al. (9) (15,726 specimens) and Weinstein et al. (10) (12,488 specimens) reported basal cell carcinoma as the most commonly biopsied entity.
Dermatopathology, unlike any other subspecialty of pathology, encompasses over 500 different diagnostic entities (11, 12). Despite this wide variety, several diagnostic entities are frequently encountered, while many others are rarely seen. In this study, 45 diagnoses accounted for an astounding 96% of all biopsies, and 15 of these diagnoses accounted for 84% of all biopsies. The remaining 4% of cases were composed of 206 diagnoses, and 250 established diagnoses were not encountered in the calendar year of study. This pattern reflects current dermatology practice in the United States, wherein the 20 most commonly encountered diseases account for 85.4% of all diagnoses made by dermatologists (9).
In the requirements set forth by the ACGME, dermatopathology fellows should examine at least 5,000 dermatopathology specimens during fellowship (13). Based on the findings of this study, that requirement may be insufficient: even in a sample of 85,000 specimens examined in an academic institution, over 250 established diagnoses were not encountered in a calendar year. Furthermore, fellows in dermatopathology who only review the minimum required number of specimens may not encounter a sufficiently wide breadth of diagnoses, particularly those of inflammatory or infectious origin. In this study, inflammatory or infectious entities represented only 9.3% of all diagnoses. The ratio of neoplastic to inflammatory or infectious diagnoses reported here is consistent with a prior study, also representing practice in the United States (10). However, this pattern is likely regional, given that studies in Greece and India have described dermatopathology caseloads comprised of 55% and 75% inflammatory and infectious disorders, respectively (14, 15). These potential deficiencies in case variety can be overcome by the dermatopathology fellow with the use of study sets for unusual cases as outlined by the ACGME (13).
Although instant recognition of “bread and butter” cases is a necessity that dermatopathology fellows must train to identify when signing out a large volume of cases, ensuring that a systematic approach to diagnosis and recognition of tissue reaction patterns during training may be lost or over-looked due to the repetitive nature of the majority of cases. Additionally, for Dermatology trained dermatopathology fellows, prior familiarity and experience with molecular pathology testing and its relevance to fellowship training is lacking with reportedly about half of dermatopathology fellows not receiving adequate instruction per their fellowship directors (16).
5.1. Conclusions
A limited number of dermatopathology diagnoses, with a heavy predominance of neoplasms, is seen in practice, with 45 diagnoses accounting for 96% of biopsy specimens examined, consistent with current dermatology practice in the United States. As a diagnostic group, melanocytic nevi (including dysplastic nevi) represent the most common reason for biopsy, followed by seborrheic keratosis, basal cell carcinoma, and squamous cell carcinoma. The required minimum number of reviewed specimens in ACGME-accredited dermatopathology fellowships may be reconsidered, given that a large number of established but uncommon, inflammatory, and infectious disorders comprise less than 4% of a large academic dermatopathology sample. Consideration of a national digital slide share program between ACGME accredited dermatopathology departments can serve to distribute unusual cases between institutions and facilitate educating fellows in recognition of an uncommon diagnosis. Additionally, preservation of high volume within academic institutions is essential for education of dermatopathology fellows and dermatology residents. Limitations of this study include its single institution-based sample, which may limit is generalizability.