1. Background
Melasma is a skin hypermelanosis disorder caused by melanogenesis dysfunction (1, 2). The lesions are circumscribed, symmetrical brownish macules, or patches with irregular edges. It typically occurs on sun-exposed areas, especially the face (1-4). In the Southeast Asia regions, melasma accounts for 2.5% - 4% of patients seen in dermatology clinics (5).
Melasma is more commonly observed in women of reproductive age (2, 3). The severity of melasma can be assessed using the modified Melasma Area Severity Index (mMASI) (6). The mMASI scoring system was validated in 2017 and is a reliable tool to assess the severity of melasma (7). Melasma more frequently affects women. Although generally not harmful, melasma can cause significant emotional impacts on patients, such as feeling bothered, frustrated, embarrassed, and depressed about their skin appearance. The disease also causes them to feel unattractive. Therefore, it is important to properly diagnose and treat the disease to minimize the burden on the quality of life (8).
Ultraviolet radiation is generally believed to be the main precipitating factor for melasma, which is indicated by the location of the lesions that are most common in sun-exposed areas (9). Chronic exposure to UV radiation increases the production of Reactive Oxygen Species (ROS) and oxidative stress (10, 11). The amount of oxidative stress can be assessed through the measurement of molecular biomarkers, one of which is blood glutathione levels (12).
Glutathione also has anti-melanogenic effects through the inhibition of the tyrosine enzyme and convert of the eumelanin to pheomelanin (13). The predominant form of glutathione in the blood is GSH, and it is mostly found inside erythrocytes (14). The erythrocyte GSH level can be used as a valid oxidative stress biomarker (15).
2. Objectives
This study aimed to determine the correlation between erythrocyte glutathione levels and the severity of melasma.
3. Methods
A case series study was done from May to July 2018 in the Outpatient Clinic of Dermatology and Venereology Department, Dr. Mohammad Hoesin General Hospital Palembang. During the study period, 30 female patients met the inclusion criteria. The study protocol was approved by the Ethics Committee, Faculty of Medicine, Sriwijaya University.
The study used a consecutive sampling method, with a sample of melasma patients visiting the outpatient clinic during the study period. The severity of melasma was assessed based on the mMASI score. The assessment was done by researchers and supervised by board-certified dermato-venereologists.
Blood specimens were collected at the Prodia Clinical Laboratory. The erythrocyte GSH level was measured using Bioxytech GSH-420® reagent and EZ Read 2000® microplate reader with the quantitative colorimetry method.
The frequency and distribution of data were analyzed with univariate analysis and presented in the form of tables and graphs. The correlation between erythrocyte glutathione levels and the severity of melasma was tested using Spearman Rho’s test. The data analysis was performed using SPSS version 22.0.
4. Results
This study included 30 female patients with melasma who visited the Dermatology and Venereology DV clinic during the study period from May to July 2018.
Table 1 shows that the mean age of the subjects was 47.10 ± 8.16 years (range 29 to 56 years), with the majority of the patients being in the age group of 46 - 55 years (60%). Most participants had bachelor degrees (56.7%), with the majority being government employees.
| Characteristics | No. (%) |
|---|---|
| Age, y | |
| Mean ± SD | 47.10 ± 8.16 |
| Median (min - max) | 49.5 (29 - 56) |
| Age, y | |
| 26 - 35 | 4 (13.3) |
| 36 - 45 | 6 (20.0) |
| 46 - 55 | 18 (60.0) |
| 56 - 65 | 2 (6.7) |
| Education level | |
| No schooling | 1 (3.3) |
| Junior high school | 1 (3.3) |
| Senior high school | 11 (36.7) |
| Bachelor degree | 17 (56.7) |
| Occupation | |
| Unemployed | 6 (20.0) |
| Government employees | 20 (66.7) |
| Daily workers | 4 (13.3) |
The mean mMASI score was 8.72 ± 5.14, with a range of 2.0 to 16.9. The distribution of melasma severity was mild (46.7%), moderate (33.3%), and severe (20%). The most frequently encountered melasma pattern was centrofacial (56.7%), mostly distributed in the epidermal type (40%). All study participants did not report sunscreen use, and 60% of respondents had a family history of melasma. Data on the distribution of clinical characteristics are shown in Table 2.
| Characteristics | No. (%) |
|---|---|
| mMASI score | |
| Mean ± SD | 8.72 ± 5.14 |
| Median (min-max) | 8.1 (2.0 - 16.9) |
| Melasma degree | |
| Mild | 14 (46.7) |
| Moderate | 10 (33.3) |
| Severe | 6 (20.0) |
| Melasma pattern | |
| Centrofacial | 17 (56.7) |
| Malar | 10 (33.3) |
| Mandibular | 3 (10.0) |
| Type of Melasma | |
| Epidermal | 13 (43.3) |
| Dermal | 6 (20.0) |
| Mixed | 11 (36.7) |
| Sunscreen usage | |
| Yes | 0 (0) |
| No | 30 (100) |
| Family history of Melasma | |
| Yes | 18 (60.0) |
| No | 12 (40.0) |
| Erythrocyte GSH level | |
| Mean ± SD | 3.13 ± 1.05 |
| Median (min - max) | 2.94 (1.57 - 5.40) |
The mean erythrocyte GSH level was 3.13 ± 1.05 μmol/g, with a minimum value of 1.57 μmol/g and a maximum value of 5.40 μmol/g. The highest level of erythrocyte GSH was found in the age group of 35 - 45 years, which was 4.12 ± 1.09 μmol/g. The lowest was found in the age group of 46 - 55 years, which was 2.81 ± 0.99 μmol/g (Table 3). The mean erythrocyte GSH levels in the mild, moderate, and severe melasma groups were 4.05 ± 0.63, 2.63 ± 0.43, and 1.79 ± 0.16 μmol/g, respectively.
| Age | Total (N = 30) | Mean ± SD |
|---|---|---|
| 26 - 35 | 4 | 3.29 ± 0.91 |
| 36 - 45 | 6 | 4.12 ± 1.09 |
| 46 - 55 | 18 | 2.81 ± 0.99 |
| 56 - 65 | 2 | 3.13 ± 0.68 |
| Mild | 14 | 4.05 ± 0.63 |
| Moderate | 10 | 2.63 ± 0.43 |
| Severe | 6 | 1.79 ± 0.16 |
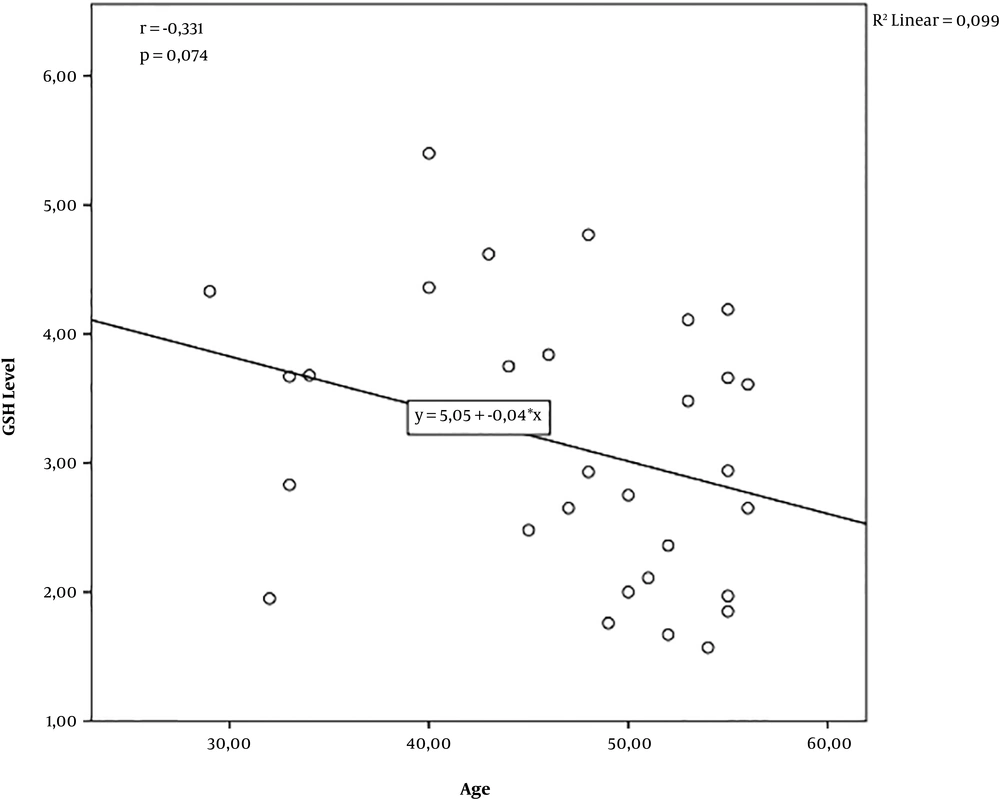
There was a negative correlation between GSH levels and age (r = -0.331; P = 0.074; n = 30). However, the correlation did not reach statistical significance. The analysis showed that older participants were found to have lower GSH levels, but the difference was not statistically significant. The R2 value of 0.099 indicated that age explained 9.9% of the variance in the GSH levels (Figure 1).
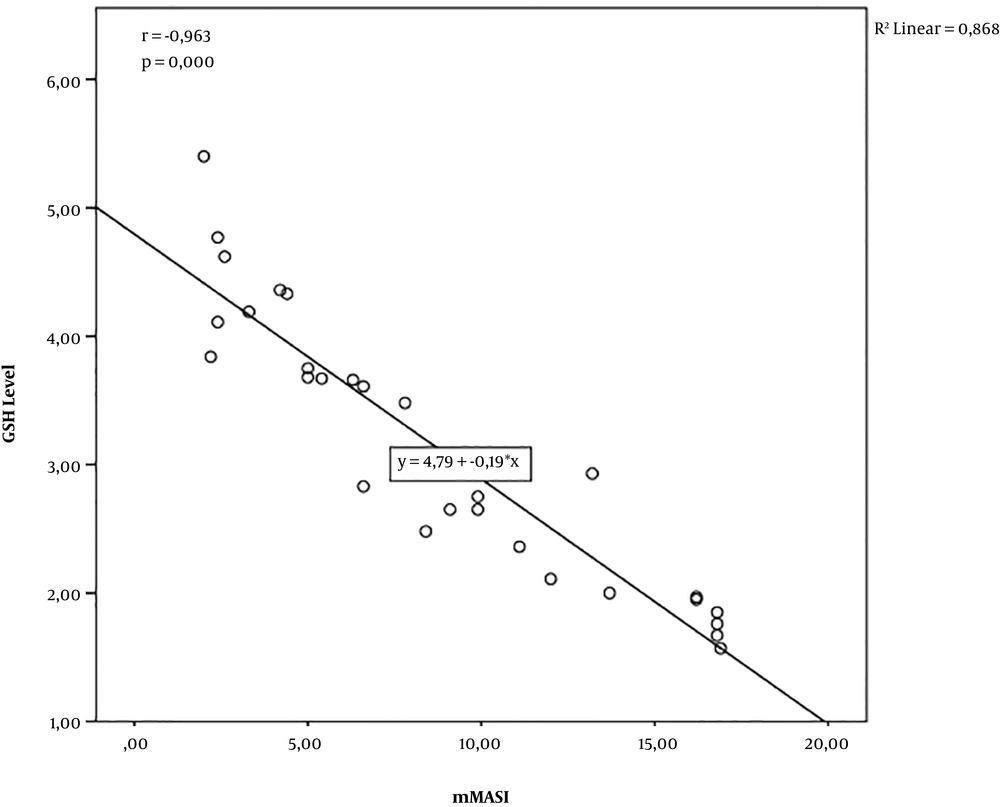
In addition, there was a significant negative correlation between erythrocyte GSH levels and mMASI score (r = -0.963; P = 0.000; n = 30), which indicated that the lower the erythrocyte GSH level, the higher the mMASI score. The R2 value was 0.868, which indicated that the erythrocyte GSH level explained 86.8% of the variance in the mMASI score (Figure 2).
5. Discussion
In this study, 30 melasma patients who met the inclusion criteria were assessed. The mMASI scores and erythrocyte glutathione levels in venous blood were determined for the participants. This study only included female patients because more than 90% of melasma patients are female (3). From 2013 to 2017, there were 240 visits by melasma patients in the DV clinic of Dr. Muhammad Hoesin Hospital, with a ratio of female to male of 27:1 (16). Epidemiological research in the Department of Dermatology and Venereology Clinic at Dr. Cipto Mangunkusumo Hospital, Jakarta, in 2004 reported that the gender distribution of melasma patients was 97.93% females to 2.07% males (17). Epidemiological studies by Hexel et al. of 953 melasma patients in Brazil obtained a ratio of female to male melasma patients of 39:1 (18).
The greater female ratio in melasma patients is explained by the influence of female sex hormones (estrogen and/or progesterone) in the pathogenesis of melasma. Several studies report that estrogen increases the expression of Trp-1, Trp-2, and tyrosinase activity in human melanocyte culture. Estrogen has shown to increase melanin synthesis in healthy human melanocytes by activating the cAMP protein kinase (PKA) pathway and upregulation of tyrosinase expression and Microopthalmiaassociated Transcription Factor (MITF) (19).
In this study, the mean age of the melasma patients was 47.10 ± 8.16 years, with a range of 29 to 56 years, mostly in the age group of 46 - 55 years (60%). The results of this study are supported by the study by Zainuddin et al., which found that the majority of the melasma patients in Makassar were over the age of 40 (76.2%) (20). Other studies found similar results. Epidemiological studies by Goh and Dlova of 205 melasma patients in Singapore discovered a mean age of 42.3 years for melasma patients (2). A cross-sectional study conducted by Ortonne et al. at nine clinics across several countries found that the mean age of melasma patients was 45.0 ± 10.7 in the United States, 41.0 ± 7.46 in France, 35.1 ± 7.18 in Germany, 40.7 ± 8.86 in the Netherlands, 39.5 ± 7.77 in Mexico, 41.3 ± 5.91 in Italy, 48.7 ± 6.71 in Singapore, 37.5 ± 9.33 in South Korea, and 48.7 ± 7.83 in Hong Kong, almost all of which are above the age of 40 (21).
The most frequently encountered melasma distribution pattern in this study was centrofacial (56.7%), which corresponds to literature and previous studies, indicating the centrofacial distribution of most melasma lesions (3). Tamega et al.’s study stated the same theory, by reporting a rate of 69.2% (22). Achar and Rathi’s studies reported that the most widespread melasma distribution was centrofacial (55.4%) (22). Research by Guinot et al. on 197 melasma patients in Tunisia also found that 76% of melasma patients experienced a centrofacial distribution pattern (23).
In this study, the type of melasma was assessed with a Wood lamp examination (UV light wavelength 340 - 400 nm) and dermoscopic examination. The Wood lamp determines the type of melasma by assessing the depth of melanin deposits in the skin (24). The most frequent type of melasma was the epidermal type (43.3%), which was in line with Zainuddin et al.’s study (78.6%) (21). A cross-sectional study in 2016 by Jagannathan et al. on 100 melasma patients in India also found the same results (47%) (24). On theory, during a dermoscopic examination, the epidermal type shows an image of regular brown pigment tissue. The dermal type shows irregular color pigments and blue-gray color. In mixed-type melasma, there are both features of the epidermal and dermal types (24).
From this study, it can be concluded that there is an inverse relationship between erythrocyte glutathione levels and the severity of melasma in melasma patients. The lower the erythrocyte glutathione level, the more the patient’s melasma severity. Since the decrease in glutathione levels can be caused by oxidative stress, it can also be concluded that an increase in oxidative stress would aggravate the severity of melasma. This is also supported by a study by Seckin et al., which found that oxidative stress, which was proven by the disrupted balance between oxidants and antioxidants, was significantly correlated with the incidence of melasma (10) However, the study did not compare the degrees of the disease.
A decrease in glutathione can cause increased hydroxylation of tyrosinase to dopaquinone, decreased speed of reaction of dopaquinone with thiol, and decreased level of thiol-dopa conjugates. These reactions cause dopaquinone to release and increase the synthesis of eumelanin in the process of melanogenesis. A reduction in glutathione also results in the accumulation of H2O2, which causes lipid peroxidation, increases tyrosinase activity, and further stimulates the melanin synthesis process (13). On the other hand, glutathione, as a strong antioxidant, is used for the treatment of melasma because of its antimelanogenic properties. The most popular routes of administration are oral, topical, and intravenous, which may be initiated for 10 weeks. Some adverse effects of glutathione administration are cutaneous rash (from a mild rash to fatal Steven-Johnson syndrome or toxic epidermal necrolysis), severe abdominal pain, thyroid, liver, or kidney dysfunction, or some lethal complications such as air embolism, blood-borne infections, and potentially fatal sepsis (25). Therefore, although promising, its usage is to be done with caution for the patient’s safety.
5.1. Conclusion
Based on the results of the analysis and discussion in this study, it can be concluded that the majority of the melasma patients (46.7%) experienced mild disease with a mean erythrocyte GSH level of 3.131 ± 1.053 μmol/g. There is an inverse relationship between erythrocyte glutathione levels and the severity of melasma. The lower the level of erythrocyte glutathione, the more the melasma severity.