1. Background
Vitiligo is a common acquired depigmenting disorder that affects between 1 and 2% of the world population to varying degrees. Vitiligo is characterized by well-defined areas of depigmentation due to loss of melanocytes (1). Despite the benign nature and course of the disease, it bears a significant impact on the social and psychological domains of the patients’ life, which in turn causes low self-esteem and poor body image (2). This perceived as well as actual stigmatization are likely to be more apparent in countries like India, where predominantly darker skin types are prevalent. (1) There are a number of factors that play a key role in the psychological impact of this cosmetically disfiguring disorder. Despite this surmise, quality of life (QoL) remains a relatively neglected aspect of most routine management strategies. Although studies evaluating these consequences of vitiligo are abundant in literature (1, 3, 4), determinants of QoL are likely to vary in different settings. Thus, the factors found to be most pertinent in a particular region can be borne in mind, thereby facilitating the adoption of a customized treatment approach, with multidisciplinary intervention, if required. Data concerning the QoL of vitiligo patients in western India are scanty.
2. Objectives
This study aims to assess the correlation between the socio-demographic and disease-related variables and their impact on the quality of life in patients with vitiligo at a large tertiary care teaching hospital in Western India.
3. Methods
A cross-sectional observational study was performed at Byramjee Jeejeebhoy Government Medical College and Sassoon General Hospitals, Pune, Maharashtra, India. Sixty patients (above 16 years of age) with a definitive diagnosis of vitiligo were enrolled over six months (August 2019-February 2020) after obtaining institutional ethics committee approval. Informed consent was obtained from all adult participants or parents/legal guardians of minors with strict adherence to norms of confidentiality and good clinical practice. Basic demographic data (including the age, sex, address) and duration of disease were elicited from the patient. The socioeconomic class was determined by the Modified Kuppuswamy Scale (5). The QoL was measured using a predesigned, validated questionnaire, Dermatology Life Quality Index (DLQI) (6), which was translated and administered in vernacular (Marathi/Hindi) language. Patients unable to fill the questionnaire were interviewed verbally, and their responses were noted. The final overall score of the questionnaire is interpreted as follows: 0-1: no effect on patient’s life; 2 - 5: small effect on patient’s life; 6 - 10: moderate effect on patient’s life; 11 - 20: very large effect on patient’s life or 21 - 30: extremely large effect on patient’s life. For DLQI, the higher the score, the lower is the QoL. The type of vitiligo (stable/unstable)- was assessed with the help of a VIDA score (Vitiligo disease activity score). The VIDA is a six-point scale for assessing vitiligo activity. Active vitiligo is defined as either expansion of existing lesions or the appearance of new lesions. Grading is as follows: VIDA Score +4 - Activity of 6 weeks or less duration; +3 - Activity of 6 weeks to 3 months; +2 - Activity of 3 - 6 months; +1 - Activity of 6 – 12 months; 0 - Stable for 1 year or more; and -1 - Stable with spontaneous re-pigmentation since 1 year or more (7). The Extent of vitiligo was determined by the percentage of body surface area (BSA) involvement. Detailed history-taking and clinical examination were performed for the presence of any other associated comorbidities like thyroid disorders, diabetes mellitus, alopecia areata, pernicious anemia, psoriasis, etc.
3.1. Statistical Analysis
All data were double-checked and entered into MS Excel Software. Then, data were analyzed using SPSS version 16. Quantitative data were described using the mean, median, and standard deviation. For comparison of categorical variables, an unpaired t-test was used. Body surface area and duration of disease were correlated with DLQI using Spearman's rank correlation coefficient and Pearson's correlation coefficient, respectively. Statistical significance was considered when P-value < 0.05 with a confidence interval of 95%.
4. Results
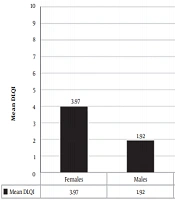
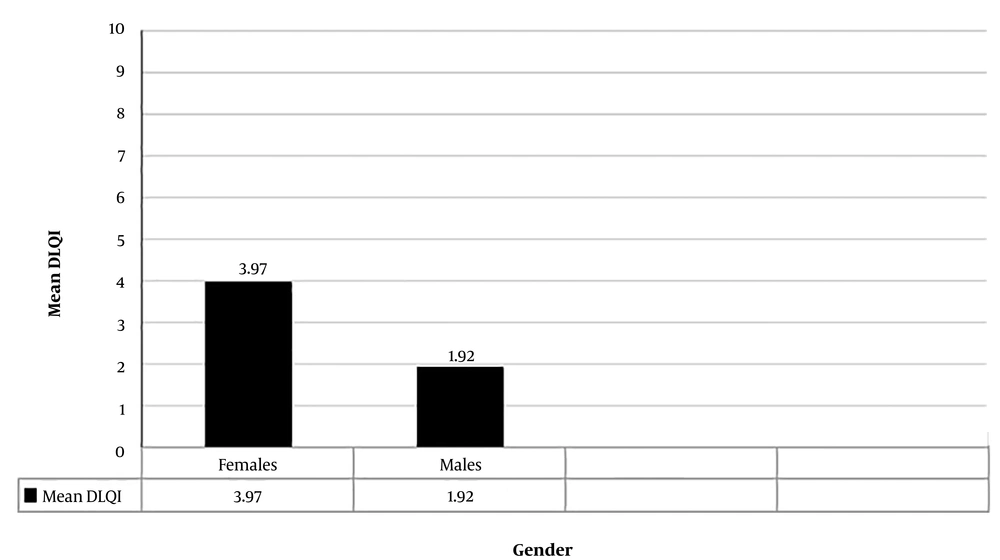
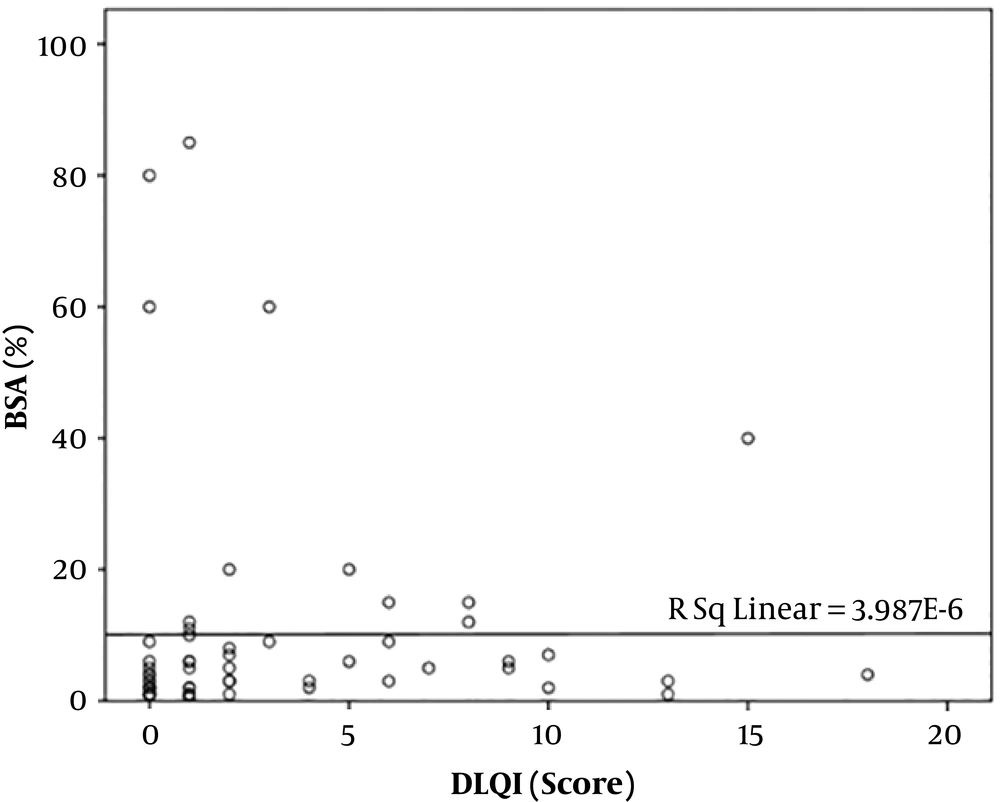
In total, data of 60 patients were fully recorded. The mean DLQI score of participants was 3.15 ± 4.2. Demographic information is tabulated in Table 1. The mean age of the study sample was 38.35 ± 16.30 years, while the oldest and youngest participants were 90 and 16 years, respectively. There was no significant correlation between age and DLQI score (P-value = 0.454). In the present study, 40% (24 subjects) of participants were male, and 60% (36 subjects) were female. The mean DLQI score in females (3.97) was higher than in males (1.92), and the difference was statistically significant (P = 0.046) (Figure 1). Also, 34 patients (56.67%) had a duration of disease (defined as an arbitrary number of years since the onset of symptoms until enrolment in the study) less than 10 years, and 21 patients (35%) had vitiligo for 11 - 20 years, while the remaining 5 patients (8.33%) had the disease for more than 20 years. A very weak negative correlation was found between the duration of vitiligo and DLQI score, but it was not statistically significant (Pearson’s correlation coefficient = - 0.010, P = 0.94). The majority of the patients (80%) had body surface area involvement of less than 10%. There was a positive correlation between body surface area affected and DLQI score (Spearman's correlation coefficient = 0.306), and it was statistically significant (P = 0.018) (Figure 2). Fourteen patients (25.33%) belonged to the Kuppuswamy socioeconomic class II, 20 (33.33%) belonged to class III, and 19 patients (31.67%) belonged to class IV. There was no significant correlation between socioeconomic status and QoL (P = 0.058). Of 60 patients, 26 (43.33%) had a VIDA score of 4+ while 14 (25.33%) had a VIDA score of zero. A very weak positive non-significant correlation was observed between vitiligo disease activity by VIDA score with DLQI (Spearman’s correlation coefficient = 0.082, P = 0.531). Among 60 patients, 5 had other comorbidities, namely diabetes mellitus (2 patients), hypothyroidism (1 patient), alopecia areata (1 patient), and psoriasis (1 patient). No statistically significant difference was noted between the mean DLQI of the patients with and without co-morbidities (P = 0.462). All enrolled patients were under treatment for vitiligo. Treatment modalities included topical and systemic medications and photochemotherapy (PUVA) and narrowband UV-B phototherapy. The majority of the patients (49, 81.67%) were on a combination of topical and systemic therapies, while 9 (15%) were on topical treatment alone. This study demonstrated no significant difference between the DLQI scores among the two groups (P = 0.629). The remaining two patients were only on NBUVB phototherapy, and their DLQI scores were 0 and 1 (no significant impairment of QoL).
| Parameter | No. (%) |
|---|---|
| Age group (y) | |
| 11 - 20 | 3 (5) |
| 21 - 30 | 24 (40) |
| 31 - 40 | 13 (21.67) |
| 41 - 50 | 8 (13.33) |
| 51-60 | 3 (5) |
| 61 - 70 | 7 (11.67) |
| 71 - 80 | 1 (1.67) |
| 81 - 90 | 1 (1.67) |
| Gender | |
| Female | 36 (60) |
| Male | 24 (40) |
| MKS class | |
| I | 4 (6.67) |
| II | 14 (23.33) |
| III | 20 (33.33) |
| IV | 19 (31.66) |
| V | 3 (5) |
| VIDA score | |
| -1 | 3 (5) |
| 0 | 14 (23.33) |
| 1 | 8 (13.33) |
| 2 | 2 (3.33) |
| 3 | 7 (11.67) |
| 4 | 26 (43.33) |
| Body surface area (%) | |
| 1 - 10 | 48 (80) |
| 11 - 20 | 7 (11.67) |
| 21 - 30 | 0 (0) |
| 31 - 40 | 1 (1.67) |
| 41 - 50 | 0 (0) |
| 51 - 60 | 2 (3.33) |
| 61 - 70 | 0 (0) |
| 71 - 80 | 1 (1.67) |
| 85 - 90 | 1 (1.67) |
| Duration of disease (y) | |
| Less than 10 | 34 (56.67) |
| 11 - 20 | 21 (35) |
| 21 - 30 | 3 (5) |
| 31 - 40 | 1 (1.67) |
| 41 - 50 | 1 (1.67) |
| Presence of comorbidity | |
| Yes | 5 (8.33) |
| No | 55 (91.67) |
| Treatment modality | |
| Only topical | 9 (15) |
| Topical and systemic | 49 (81.67) |
| Only NBUVB phototherapy | 2 (3.33) |
5. Discussion
The mean DLQI of the vitiligo patients was 3.15, indicating a mild effect on the QoL. Compared to previously conducted studies in North India, this value is low. For example, Kota et al. (8) and Sangma et al. (3) reported a mean DLQI of 7.02 and 9.08, respectively. This difference can be attributed to the social awareness created through activities like poster competitions, skits, rallies, information handouts, etc. A program is conducted annually in our institute on the 25th of June (World vitiligo day) for this cause. A series of supportive groups are created to help the affected people and promote awareness among the general public (“Shweta Association” (9) is a supportive group which covers our area, that conducts a number of activities like group counseling, marriage bureau, employment programs, movies, documentaries, etc). Age and gender may act as important determinants of QoL. Poorer QoL in females and younger individuals can logically be justified as vitiligo is a cosmetically disfiguring disorder associated with the profound social stigma. Aesthetic concerns are more considerable in the afore-mentioned sections of society, which in turn causes limited choices of clothing and imposing a higher level of emotional burden with a stronger influence on their self-esteem, as they are more conscious about their appearance (10). Moreover, in Indian society vitiligo poses major hurdles for marital prospects, which further compounds the anguish experienced by them (1, 11). In the present study, females were predictably more affected than males, which is consistent with the findings reported by previous studies (3, 4, 10, 12).
However, we found no correlation between age and QoL, which is in contrast to results reported by Patvekar et al. (1) and Kota et al. (8), which based on their findings, QoL of middle-aged patients was most affected. This result may be attributed to the increasing awareness among the older population regarding their appearance and also to the prejudice endured by these patients over the years. Working older patient groups might face rejection or ridicule at their work-place due to the disorder. Moreover, if they are dependent on their family members, the added expenditure for their treatment may result in discontent among the family. Some patients may have children or grandchildren of marriageable age, and the possibility of genetic transmission and the associated hurdles is another potential source of familial discord impacting the QoL.
In the present study, most of the patients belonged to the upper-lower or lower middle and upper-middle classes of the Modified Kuppuswamy Classification. Also, there was no significant difference in the QoL of different socioeconomic classes, which is consistent with the findings of some of the previously conducted studies (10, 11). This implies that the education, occupation, or income, which define the socioeconomic class of an individual in society, do not have a discernable impact on the stigma that they perceive. It is generally assumed that the lesser educated and poorer class of patients would be facing more difficulty in being accepted by society as compared to their more affluent counterparts. However, these results indicate that the perceived stigma is similar in all sections of our society. In our study, no significant difference was found concerning the QoL of patients with and without other associated diseases. The comorbidities commonly associated with vitiligo include autoimmune thyroiditis, diabetes mellitus, Addison’s disease, systemic lupus erythematosus, and pernicious anemia (13), which all are autoimmune disorders with a protracted and debilitating course which may, in addition to vitiligo, cause further impairment in QoL. We could not find any literature assessing this parameter and hence are unable to compare our findings.
We found that the percentage of body surface area involvement was associated with poorer quality of life. These findings were consistent with other studies (1, 14, 15). In patients having extensive vitiligo, there is a greater likelihood of involvement of exposed/visible and socially or cosmetically crucial body parts. Also, in such cases, re-pigmentation is seldom complete. Frequently, the patches of re-pigmentation may be darker than the normal skin imparting an unsightly mottled appearance. Sometimes partially re-pigmented patches can render the contrast with the vitiliginous areas even more conspicuous. Thus, paradoxically, partial response to treatment may negatively influence the quality of life unless the pigment match is cosmetically acceptable (16).
Like other studies (17), we did not find a significant correlation between the stability of disease and QoL. Some studies have drawn attention to greater constraints on QoL in those who have spreading lesions or new lesions (18). There is a wide variation regarding the effect of duration of vitiligo on QoL. While some studies (e.g., Parsad et al. (19) and Radtke et al. (20)) have reported a statistically significant relationship between DLQI scores and disease duration, while others (Kent and Al-Abadie (21)) have reported contrary results, consistent with our observations (10, 19). In cases of progressive lesions, they may go on to involve cosmetically important body parts or a greater body surface area, which may result in increased anxiety that adversely affects the patient’s psyche. Thus, the lack of disease stability and greater body surface area involvement may negatively affect the QoL. Also, the appearance of new lesions or relentlessly spreading lesions despite treatment would have a demoralizing effect on the patients as they begin to realize that their disease probably might not be cured.
We did not find any significant correlation between the mode of treatment and the QoL of patients. This parameter warrants analysis as the DLQI questionnaire includes a query regarding the effect of treatment on the patient’s mental well-being. Although there are many studies that mentioned the co-relation between the response to treatment and QoL, literature regarding the effect of type of treatment modality on DLQI is limited. Patvekar et al. have reported a higher impairment in QoL among those who were treated previously, especially PUVA with or without oral or topical corticosteroids (1). The fact that those who previously received a substantial course of treatment should be further followed up indicates that they were not satisfied with the results of initial treatment, and the anguish suffered by them is enough to force them to seek medical help again. Such patients evidently have a diminished QoL and need to be counseled adequately to alleviate their anxiety, and may require psychiatric intervention and anxiolytics to be able to cope with stress. Patients with vitiligo often suffer financial loss and work absenteeism because they need to take time off to attend consultations and for repeated and long-term PUVA and NBUVB sessions (22). In keeping with the current concept of vitiligo as a psychosomatic disorder, mental and physical stress have been strongly implicated in their pathogenesis as well as remission and recurrence. A sizeable proportion of these patients are on systemic immunosuppressive treatment, like corticosteroids, azathioprine etc., with well-established potential for adverse effects. Therefore, more attention should be paid to this issue.
We attempted to compare our findings with other studies. The observed discrepancy in the findings can be attributed to factors such as study design and applied QoL tools. Patvekar et al. conducted a cross-sectional questionnaire-based study in western India, including a hundred patients of vitiligo (1), using the Vitiligo Impact Scale-22 (VIS-22) questionnaire, which is a disease-specific, modified version of VIS questionnaire (1). They reported no gender-related difference in QoL and a negative correlation between QoL and age, thus underlining the effect of study design and methods on the results (1). Hedayat et al (using VitiQoL questionnaire on 173 patients) showed a higher impairment in QoL in women, younger age groups, and patients with disease duration between 5 to 20 years (10). Though vitiligo has a profound psychological impact, it is largely asymptomatic and therefore, the general dermatological questionnaires (like DLQI) may not be able to elicit the impact appropriately. Hence vitiligo-specific questionnaires are recommended. This issue is illustrated by Kota et al., wherein the patients were administered three questionnaires, namely DLQI, VIS-22, and QIDSSR16 (Quick Inventory of Depressive Symptomatology) (8). It was seen that the DLQI did not show any difference in the QoL among different age groups while the VIS-22 showed a significantly higher score in the younger (18 - 30 years) age group (8).
Investigating a large number of patients and including vitiligo-related parameters using DLQI (which is one of the most widely used and validated personal satisfaction surveys) are among the strengths of the present study. The current study had limitations, including not having a control group (as DLQI is applicable only for patients and not for healthy individuals) and using a general, rather than a vitiligo-specific, questionnaire, which might explain why many variables demonstrated a weak co-relation with DLQI.
5.1. Conclusion
The small effect on DLQI found in our study testifies to the success of the public awareness campaigns, self-help groups, and non-government organizations operational in our region, which have brought about a positive change in the attitude of patients and society alike. Among the two factors found to have a significant effect on the QoL in vitiligo in our study, gender was a non-modifiable parameter. However, the stigma associated with the disease in society may be alleviated by creating patient as well as public awareness. Body surface area involvement may be controlled by instituting early treatment, thus preventing progressive depigmentation. There is an immense amount of literature regarding the QoL of vitiligo patients from different parts of India as well as the world with conflicting observations. This can be attributed to regional variations and the large number of disease- and patient- related determinants along-with some occult variables that have not been hitherto analyzed. Therefore, vigilance must be exercised, irrespective of questionnaire results, for any subtle signs indicating QoL impairment to enable timely action. Counseling and possible psychiatric intervention may be needed in these patients to allay their suffering. QoL is an important and useful tool that deserves a place in the armamentarium for the holistic management of vitiligo.