1. Context
Elderlies are more susceptible to degenerative diseases including neurodegenerative diseases. The most common neurodegenerative disease among elderlies is Alzheimer’s disease (AD) (1). Alzheimer’s disease has always been a challenge in healthcare since it is the main cause of dementia, with 60 - 80% of AD patients suffering from dementia (2, 3). The incidence of AD is high and keeps rising every year (4). According to the World Alzheimer Report, there were about 40 million cases of AD in 2015. About US$ 818 billion is annually spent for research on AD, and every three seconds one new case of AS is diagnosed. The situation is further deteriorated with the 325% increase in the incidence of the disease by 2040 (5, 6).
Clinical manifestations of AD also affect the well-being of elderlies. Alzheimer’s disease may manifest with a progressive decrease in cognitive skills, language, visuospatial skills, personality, and behaviors, which may lead to the complete loss of basic daily life activities (7). These clinical manifestations are due to the pathogenesis of AD causing neurotoxicity (2, 8-11).
The pathology of AD roots in the formation of senile plaque (SP), and the accumulation of amyloid beta (Aβ) and neurofibrillary tangles (NFT) is the most common cause of SP. The formation of SP is through various cascades initiated when Aβ is present due to the mutation of the PS1/2 gene of the amyloid precursor protein (APP). This cascade involves the formation of Aβ oligomer, which causes synaptic dysfunction and neurohormonal instability. The presence of Aβ also induces the activation of inflammatory microglia and astrocytes, which play a role in local inflammation and increased oxidative stress. This leads to the alteration of kinase and phosphatase and hyperphosphorylation of tau protein (P-tau) creating NFT. Like Aβ oligomer, NFT also disturbs synaptic communication (2, 8-11).
Currently, non-pharmacological and pharmacological therapies are available. Non-pharmacological therapy utilizes various factors such as open communication between healthcare provider and patient, behavioral therapy through consistency and simplifying the environment, cognitive-behavioral therapy, light therapy, and music therapy (2, 12, 13). In pharmacological therapy, on the other hand, acetylcholinesterase inhibitors and memantine are used. However, non-pharmacological and pharmacological therapies can only maintain patient’s quality of life and are unable to reduce the severity of the disease (14).
A potential therapy for AD is utilizing adipose-derived stem cells (ADSCs), which are extracted through liposuction, a minimally invasive procedure. Besides being minimally invasive to extract, ADSCs are abundant in source because fat tissue can be found almost everywhere in the body. Another rationale for utilizing ADSCs as cellular therapy is their multipotency and autologous usage, which allows to avoid immunological rejection and tumorigenesis (15-17).
2. Objectives
This systematic review was performed to review the outcomes of treatment with adipose-derived stem cells in Alzheimer’s disease starting from in-vitro, in-vivo, and clinical trials.
3. Data Sources
3.1. Search Strategy
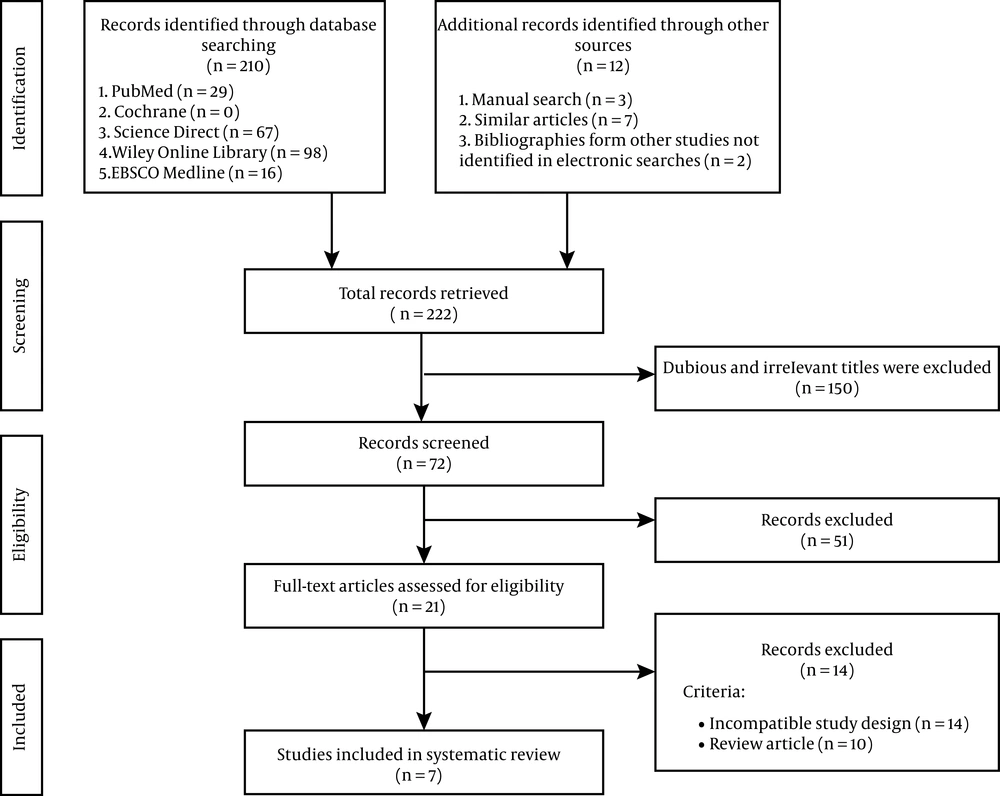
The preferred reporting items for systematic reviews and meta-analysis (PRISMA) was used to create this systematic review. Relevant studies were retrieved from PubMed, Cochrane Library, ScienceDirect, Wiley Online Library, and EBSCOhost from inception to 6 August 2020, with search terms as following: (“adipose tissue-derived stem cells” [All Fields] OR “stromal vascular fraction” [All Fields]) AND (“Alzheimer” [All Fields]). The search included preclinical studies and randomized controlled trials with no language restrictions applied. However, studies included in this review were restricted to English and Bahasa Indonesia, which were the only languages readable by the reviewers. Figure 1 shows the details of the literature search strategy.
3.2. Inclusion and Exclusion Criteria
The inclusion criteria for the included studies were: (1) study design: preclinical studies and clinical trials (either randomized or non-randomized); (2) language: English or Bahasa Indonesia; (3) comparison and intervention: ADSCs as main treatment; and (4) outcome parameter: Aβ peptide concentration, P-tau concentration, and subjects’ cognitive and spatial skills. Furthermore, the exclusion criteria comprised of (1) no extractable data and (2) irretrievable full-text articles.
3.3. Study Selection
The selection process for the included studies in the systematic review is shown in Figure 1. The initial search yielded 222 studies, of which 150 studies were irrelevant and 51 studies were excluded after screening the title and/or abstract. Furthermore, the excluded studies were based on no relevancy to the pre-defined inclusion criteria. Thus, seven studies were included in this systematic review, of which two were in-vitro studies, four were in-vivo studies, and one was a clinical trial.
3.4. Data Extraction and Quality Assessment
Literature search and data extraction were performed by two independent reviewers (GK and RMS), and disagreements were resolved through discussion. The following information was extracted from each study: author indicated by reference number, study design, intervention methods used, subjects, bioactive factors measured, outcomes, Aβ concentration, amyloid precursor protein C-terminal fragment (APP-CT) concentration, number of amyloid plaques, oxidative stress, bioactive factors, neprilysin (NEP) concentration, neurogenesis, synaptogenesis, synaptic and dendritic stability, neuron apoptosis, blood-brain barrier (BBB) penetration, anti-inflammatory microglial activation, and cognitive and spatial skills.
The two reviewers (GK and RMS) assessed the included studies’ quality. For the evaluation of the perceived risk of bias within the preclinical studies on animal model, the Systematic Review Centre for laboratory animal experimentation (SYRCLE) Risk of Bias tool was used (18), while the non-randomized phase 1 clinical study without control group was evaluated using the methodological item for non-randomized studies (MINORS) tool (19). No pre-determined guidelines were available to assess the bias risk for in-vitro studies.
4. Results
4.1. Characteristics of the Articles
The characteristics of the included studies are shown in Table 1. The studies mostly aimed to investigate the efficacy of ADSCs in AD with in-vitro and in-vivo studies also aiming to find the rationale behind the efficacy. In addition, two in-vivo studies investigated whether ADSCs could penetrate BBB of AD, and the non-randomized phase 1 clinical trial investigated the safety of ADSCs in 10 AD patients.
| Reference | Study Characteristics | Subject Characteristics | Intervention | ||||
|---|---|---|---|---|---|---|---|
| Study Design | Phase | Aims/Purpose | Subjects | Intervention Treatment Groups | Intervention Methods | Bioactive Factors | |
| (20) | In-vitro | - | To examine efficacy of ADSC-Exo in AD | Neutrosphere cells from TG2576 transgenic mice | hADSC | 72 hours co-culture | ADSC-Exo |
| (21) | In-vitro | - | To examine efficacy of ADSC-Exo (NEP) in AD | N2a cell line | hADSC | 56 hours co-culture | ADSC-Exo (NEP) |
| (22) | In-vivo | - | To examine the efficacy of ADSC in activating the alternative phenotype microglia of AD | APP/PS1 transgenic mice | mADSC | Intracerebral injection | IL-4 and Arginase I |
| (23) | In-vivo | - | To examine the beneficial effects and neurogenesis of ADSC in AD | APP/PS1 transgenic mice | mADSC | Intrahippocampal injection | ADSC-Exo |
| (24) | In-vivo | - | To examine if ADSC could penetrate BBB; efficacy of ADSC in the early (intracerebral injection) and late stage (intravenous injection) of AD; efficacy of ADSC in preventing AD | APPswe TG2576 transgenic mice | hADSC | Intracerebral injection; intravenous injection | ADSC-Exo (NEP), IL-10, and VEGF |
| (25) | In-vivo | - | To examine if ADSC could penetrate BBB | TG2576 transgenic mice | hADSC | Intravenous injection | - |
| (26) | Non-randomized clinical trial | I | To examine safety and efficacy of ADSC in AD patients | 10 Alzheimer patients | SVF | Subgaleal ommaya reservoir | - |
Study Characteristics
4.2. Risk of Bias within Studies
Pre-clinical studies in animal model were assessed using the SYRCLE Risk of Bias tool. As for the preclinical in-vivo studies on animal models, four preclinical studies were rated on the perceived risk of bias using SYRCLE tool. For two of them, the allocation sequence was reported unclearly, raising concern for selection bias. Moreover, all the studies were not documented for blinding housing, blinding intervention, and blinding outcome assessment. These would raise some concern regarding the potential for performance bias and detection bias. Lastly, the included in-vivo studies were free of risk of reporting and other biases. The summary of risk of bias for preclinical studies in animal models is presented in Table 2.
| Type of Bias and Items | SYRCLE’s Tool for Assessing Risk of Bias (18) | |||||
|---|---|---|---|---|---|---|
| Domain | Question | Reference | ||||
| (22) | (24) | (25) | (23) | |||
| Selection bias | ||||||
| 1 | Sequence generation | Was the allocation sequence adequately generated and applied? | Yes | Yes | Unclear | Unclear |
| 2 | Baseline characteristics | Were the groups similar at baseline or were they adjusted for confounders in the analysis? | Yes | Yes | Unclear | Unclear |
| 3 | Allocation concealment | Was the allocation adequately concealed? | No | Yes | Yes | Yes |
| Performance bias | ||||||
| 4 | Random housing | Were the randomly animals housed along the experiment? | Unclear | No | Unclear | Unclear |
| 5 | Blinding | Were the caregivers and /or investigators blinded from knowledge which intervention each animal received during the experiment? | No | Unclear | Unclear | No |
| Detection bias | ||||||
| 6 | Random outcome assessment | Were animals selected at random for outcome assessment? | Unclear | No | Unclear | No |
| 7 | Blinding | Was the outcome assessor blinded? | Unclear | No | No | No |
| Attrition bias | ||||||
| 8 | Incomplete outcome data | Were incomplete outcome data adequately addressed? | Yes | Yes | No | Yes |
| Reporting bias | ||||||
| 9 | Selective outcome reporting | Was the study free of selective outcome? | Yes | Yes | Yes | Yes |
| Other | ||||||
| 10 | Bias from another sources | Is the study free of problems leads to high risk of bias? | Yes | Yes | Yes | Yes |
Risk of Bias Assessment for In-vivo Studies a
Assessment of the perceived risk of bias in the non-randomized phase 1 clinical study was performed using the MINORS Risk of Bias tool. The study did not apply a control group and was non-randomized; thus, there was no comparison or blinding. This explains the 0 score for items 8 - 11 (Table 3). It raises concern regarding the potential bias of the outcome report. Lastly, the study reported adequate statistical analysis to support the outcome documented in the study. The summary of risk of bias for the non-randomized phase 1 clinical study is shown in Table 3.
| MINORS tool for assessing risk of bias (19) | ||
|---|---|---|
| Item | Components | Duma et al. [2019] (26) |
| 1 | A purpose of study | 2 |
| 2 | Patient inclusion | 2 |
| 3 | Data collection | 2 |
| 4 | Goal of study related to the purpose | 2 |
| 5 | Assessment of study unbiased | 2 |
| 6 | Period of patient follow-up | 2 |
| 7 | Losing of patient follow up (< 5%) | 2 |
| 8 | The size study size | 0 |
| 9 | Group of control | 0 |
| 10 | Contemporary groups | 0 |
| 11 | Groups baseline | 0 |
| 12 | Analysis of statistics | 1 |
| Total Score | 15 | |
Risk of Bias Assessment for Non-randomized Clinical Trial Study a
4.3. Efficacy
Effects of ADSCs on AD (Table 4) were evaluated by two in-vitro studies, four in-vivo mouse model studies, and one clinical trial. Outcomes extracted for assessment include Aβ concentration, P-tau concentration, APP-CT concentration, number of amyloid plaques, oxidative stress, bioactive factors, NEP concentration, neurogenesis, synaptogenesis, synaptic and dendritic stability, neuron apoptosis, BBB penetration, activation of alternative phenotype microglia, cognitive skills, and spatial skills.
| Reference | Outcomes | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aβ Concentration | P-Tau Concentration | APP-CT Concentration | Number of Amyloid Plaques | Oxidative Stress | Bioactive Factors | Neprilysin Concentration | Neurogenesis | Synaptogenesis | Synaptic And Dendritic Stability | Neuron Apoptosis | Blood-Brain Barrier Penetration | Alternative Phenotype Microglia | Cognitive Skills | Spatial Skills | |
| (20) | Decreased (P < 0.01) | - | - | - | - | - | - | Increased (P < 0.01) | Increased (P < 0.01) | - | Decreased (P < 0.01) | - | - | - | - |
| (21) | Decreased (intracellular and extracellular) (P < 0.001) | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| (22) | Decreased (hippocampus: P < 0.01; cortex: P > 0.05) | - | - | Decreased (hippocampus: P < 0.01; cortex: P < 0.05) | - | Decreased mRNA expression of TNF-α (hippocampus: P < 0.01; cortex: P < 0.05) and IL-1β (hippocampus: P < 0.01; cortex: P < 0.05); increased mRNA expression of IL-4 (hippocampus and cortex: P < 0.01) and ARG1 (hippocampus and cortex: P < 0.01); increased mRNA expression of IDE (hippocampus and cortex: P < 0.01) and MMP-9 (hippocampus: P < 0.01; cortex: P > 0.05) | Increased (P < 0.01) | - | - | - | - | - | Activated (P < 0.01) | Increased (P < 0.05) | - |
| (23) | - | - | - | - | Decreased (P < 0.01) (hippocampus) | - | - | Increased (P < 0.01) | - | - | - | - | - | Increased (P < 0.05) | - |
| (24) | Decreased (IC: P < 0.05; IV: P < 0.05) | - | Decreased (IC: P < 0.05; IV: P < 0.05) | Decreased (IC/cortex: P < 0.05; IC/hippocampus: P < 0.05; IV/cortex: P < 0.05; IV/hippocampus: P < 0.14) | - | Increased IL-10 (IC and IV: P < 0.05) and VEGF (IC 6 weeks: P < 0.01; IC and IV 4 months: P < 0.05) | Increased (IC: 1.29-fold, P < 0.05; IV: 1.14-fold, P < 0.05) | Increased (IC: P < 0.05; IV: P > 0.05) | - | Increased | Decreased (P < 0.05) | Penetrated | Activated | - | Increased (IC: P < 0.01; IV: P < 0.05) |
| (25) | - | - | - | - | - | - | - | - | - | - | - | Penetrated | - | - | - |
| (26) | - | Decreased (3 patients) | - | - | - | - | - | Increased (2 patients had an increase of hippocampal volume) | - | - | - | - | - | Increased (5 patients) | - |
Reported Outcomes Related to the Efficacy of ADSCs in AD
Overall, in-vitro and in-vivo studies showed a significant reduction in Aβ concentration, APP-CT concentration, number of amyloid plaques, oxidative stress, neuron apoptosis when exposed to Aβ, and mRNA expression of TNF-α and IL-1β. A fa fall in P-tau was observed in three patients in the non-randomized phase 1 clinical trial. On the other hand, in-vitro and in-vivo studies exhibited a significant increase in IL-4 mRNA expression, IL-10, vascular endothelial growth factors (VEGF), and NEP production, neurogenesis, synaptogenesis, synaptic and dendritic stability, cognitive skills, and spatial skills. The non-randomized phase 1 clinical trial also showed an improvement in cognitive skills in five patients and an increase in hippocampal volume in two patients. An intriguing finding was the ability of ADSCs to penetrate the BBB of AD patients and activate the alternative phenotype microglia.
5. Discussion
The regenerative potential of ADSCs has been studied and used for osteochondral tissue engineering and cell therapies, such as wound healing and calvarial defects (27). Based on the systematic review, we found that ADSCs also have a potential to be used in cellular therapy for AD.
The potential of ADSCs to be used as a regenerative cellular therapy for AD lies in their multipotency and autologous usage. This enables ADSCs to create any tissue in the body, except placenta, and avoid immunologic rejection. The differentiation of ADSCs is usually induced when they receive certain signals from damaged tissue that make them acquire tissue-specific phenotypes. Aside from pluripotency, the regenerative effects of ADSCs lie mainly in their autocrine production of various bioactive factors, such as growth factors, cytokines, chemokines, antiapoptotic factors, and immunomodulators in the form of gene products such as mRNA and miRNA species (17). These bioactive factors are often secreted in the form of exosomes and may affect the behavior of affected cells. Furthermore, bioactive factors which have immunomodulatory effects result in positive effects on AD, which is worsened through neuroinflammation in the CNS (17, 28).
The reviewed studies showed that ADSCs increased neurogenesis, synaptogenesis, and the stability of synapses and dendrites. The rationale behind this finding mostly lies in the multipotency of ADSCs, exosomes produced, and their ability to penetrate the BBB. Differentiation ADSCs can be induced by reactive oxygen species (ROS). In AD, excessive ROS induced by elevated levels of Aβ peptide leads to oxidative stress and inappropriate microenvironment for neurogenesis. Although ROS can induce differentiation of neural stem cells (NSC) or ADSCs, oxidative stress results in toxicity and cell death of the newly differentiated neurons. Interestingly, ADSCs have the ability to reduce ROS and increase the self-renewal capacity of the CNS that leads to neurogenesis through NADPH oxidase and PI3K/Akt pathway (23, 29). The possible mechanism behind ADSCs reducing ROS is through the exosomes produced, which may decrease ROS generation, enhance ROS’ scavenging capability, or repair oxidized molecules (23). Furthermore, reduced ROS also explains that increased neurogenesis promotes synaptogenesis and prevents neuronal membrane damage, which leads to the enhanced stability of both synapses and dendrites (30).
Aside from ROS reduction, exosomes of ADSCs (ADSC-Exo) have been found to induce remyelination in damaged neurons, which also explains the neuroregenerative capability of ADSCs (31). Moreover, ADSC-Exo have been reported to upregulate VEGF.26 It has been reported that VEGF plays a role in neuronal wiring of the CNS by regulating neuronal cell migration and neuroregeneration, which explains the increased neurogenesis, synaptogenesis, and stability of synapses and dendrites (32).
The ability of ADSCs to reduce ROS in AD also explains the reduced neuron apoptosis and increased neuron viability observed (24). Reports on the upregulation of VEGF by ADSC-Exo also explain the decrease in neuron apoptosis and increase in neuron viability. Vascular endothelial growth factors are reported to be able to inhibit Aβ-induced cytotoxicity in neurons. In AD, amyloid plaques co-aggregate with the VEGF produced. This causes VEGF depletion and neurodegeneration due to reduced neuroprotective effects (32). This phenomenon is further explained by the ability of ADSC-Exo to exert their neuroprotective effects against glutamate excitotoxicity, which causes neurodegeneration in AD. The mechanism through which ADSC-Exo exert their neuroprotective effects is the amelioration of neural recovery marker. The neural recovery marker ameliorated is the growth-associated protein 43 (GAP-43) and the number of GAP-43 positive neurites, which leads to neural development and regeneration. Another mechanism of ADSC-Exo is through increasing the levels of ATP, NAD, and NADH, which rescues neurons from glutamate-induced neuronal energy depletion in AD (33-35).
The reviewed studies also reported the activation of the alternative phenotypes of microglia by ADSCs. These studies reported this finding through the observation of strong expression of IL-4 and Arg-1, which are the markers of alternative phenotype microglia. This activation is beneficial because they play a protective role against AD by mediating the clearance of Aβ and reducing the production of proinflammatory and neurotoxic cytokines, which does not worsen the pathological course of AD. the clearance of Aβ is mediated through the production of IL-4 and IL-10, which are neuroprotective and through the phagocytosis of Aβ by alternative phenotype microglia (36). The production of IL-4 by alternative phenotype microglia further activates alternative phenotype microglia (37). On the other hand, the production of IL-10 is due to its ability in neutralizing cytotoxic inflammatory processes induced by Aβ and down-regulating IL-1β and TNF-α synthesis (38). The down-regulation of pro-inflammatory cytokines is beneficial towards the phagocytic ability of alternative phenotype microglia, which is attenuated by pro-inflammatory cytokines (39).
Furthermore, the degradation of Aβ is not only by phagocytosis through microglia, but ADSC-Exo also play a role in the degradation of Aβ. It has been found that ADSC-Exo stimulate increased production of Aβ degradative enzymes. These enzymes include insulin-degrading enzyme (IDE) and NEP (40, 41). Although the two of them are able to degrade Aβ both intracellularly and extracellularly, the degradative mechanisms differ between the two of them. Neprilysin degrades Aβ by cleaving peptides on the N-terminal of hydrophobic residues and has broad substrate specificity (42, 43). On the contrary, IDE is only proteolytic towards β-structure-forming substrates, which makes them the major protease responsible for Aβ clearance (41). Moreover, an increase in matrix metalloproteinase-9 (MMP-9) was also reported and may also explain the reduction of Aβ. Studies have found that MMP-9 exerts neuroprotective effects through the cleavage of APP by enhancing α-secretase activity. With the enhanced cleavage of APP, the production of Aβ will be diminished (43). Undoubtedly, the decrease in Aβ will cause reduce ROS and will also stop the formation of both P-tau and NFT, which explains a reported finding in this review (11).
Lastly, studies reviewed also reported that ADSCs alleviate cognitive and spatial skills impairment. The main cause of cognitive and spatial skills impairment is the assembly of Aβ, which causes many pathological effects (44). Adipose-derived stem cells’ ability to degrade Aβ explains this finding (40, 41).
5.1. Conclusions
In light of all the evidence presented in this systematic review, ADSCs have an excellent potential as a novel therapy for AD. Preclinical studies found that ADSCs have neuroregenerative and neuroprotective capabilities and reduce the concentration of Aβ, which plays a main role in various pathological processes in the progression of AD. These findings were further proven in another preclinical study and a non-randomized phase 1 clinical trial, which showed that ADSCs could alleviate cognitive and spatial skills impairment.