1. Context
Mucosal lichen planus (MLP) is a chronic inflammatory immune-mediated disease that affects the oral mucosa, but may also involve genitalia, ocular mucosae, esophagus, and modified epithelium and mucosae of the ear canal (1, 2). Desquamation of involved mucosae is associated with considerable pain and scarring. Involvement of tubular structures such as the lacrimal duct, esophagus, external ear canal, vagina, and urethra, can result in stenosis and organ function compromise, including persistent tearing, dysphagia, hearing loss, dyspareunia, and inability to void. Chronic mucosal inflammation may result in synechiae in ocular MLP or agglutination and complete resorption of external genital structures in genital MLP (3, 4).
MLP is estimated to affect 0.5% to 2% of the general population (5, 6). Unlike the cutaneous form of lichen planus (LP), with a tendancy to sponataneous remission, MLP is typically progressive and often recalcitrant to therapy (5). Topical treatment remains the mainstay of therapy (7), but is frequently ineffective in more severe ulcerated presentations. Systemic therapy has been used in refractory presentations, but there are no consensus treatment guidelines or US Food and Drug Administration-approved treatments for MLP. We, therefore, sought to review the literature on reported therapies, summarize current therapies, and propose a treatment algorithm based on existing evidence and personal experience.
2. Methods
An English-language literature search was conducted using PubMed, MEDLINE, and Cochrane databases to identify publications describing systemic treatment of MLP. Bibliographies of retrieved publications were also used to identify other relevant publications not included in the initial search. Key search terms included “mucosal lichen planus”, “oral lichen planus”, “lichen planus”, “genital lichen planus”, “ocular lichen planus”, “otic lichen planus”, and “esophageal lichen planus” in combination with the keyword “treatment”. Review articles and studies for topical treatment of MLP were excluded.
Ninety-three studies were reviewed, including 27 clinical trials, 11 retrospective and 6 prospective studies, 35 case reports and 16 case series using biologics, immunosuppressive therapies, immunomodulating and anti-inflammatory drugs, vitamin A derivatives, devices and interventional therapies, and nonprescription drugs.
3. Results
Reported treatments were classified into the following categories: biologics, antimetabolic immunosuppressives, direct anti-T cell agents, other immunomodulatory agents, antibiotics, retinoids, systemic corticosteroids and procedure-based interventions (Appendix 1 in Supplementary File).
3.1. Biologics
3.1.1. Rituximab
Two case reports demonstrated dramatic improvement of dysphagia and odynophagia in severe esophageal MLP with Riruximab, with complete disappearance of active LP lesions and esophageal stenosis on endoscopy at 3-month follow-up (8, 9). Imunohistochemical examination of esophageal mucosa showed disappearance of CD20+ cells. Sartori-Valinotti et al. (10) presented a 10-year Mayo Clinic experience with otic LP through a case series of 19 patients. One patient had severe LP affecting the ear, oral cavity, esophagus, and genital area, and remarkable relief was noted with rituximab prescribed primarily for Sjögren syndrome (10).
3.1.2. Tumor Necrosis Factor-α Antagonists: Adalimumab and Etanercept
Etanercept is effective in psoriasis, aphthous stomatitis and graft-versus-host disease, which share a similar pathogenesis with MLP (11). Response to adalimumab in recalcitrant MLP has been reported in 2 patients with severe orogenital involvement (12, 13) and 1 with severe erosive oral LP (OLP) experienced major symptom relief after 2 weeks of etanercept (11).
3.2. Antimetabolite Immunosuppressives: Mycophenolate Mofetil, Azathioprine, and Methotrexate
3.2.1. Mycophenolate Mofetil
A retrospective study of MLP patients (oral and genital) treated with MMF for 1.7 years demonstrated statistically significant reduction in number of lesions, disease activity, and pain in 15 of 22 patients, suggesting that MMF can be considered in MLP refractory to topical therapy (14). Frieling et al. (3) reported 3 cases of disseminated and erosive OLP, 1 with genital involvement, successfully managed with MMF for 5 months.
Wee et al. (15) retrospectively reviewed 10 patients with recalcitrant erosive OLP (including vulvovaginal and penile involvement) treated with MMF for 3.7 years. Six achieved remission, 1 had well-controlled disease, and 3 partially controlled disease.
3.2.2. Azathioprine
In a case series by Verma et al. (16), 9 patients with severe erosive OLP or generalized LP received azathioprine 2 mg/kg daily for 3 - 7 months. Therapeutic response was determined by flattening or healing of lesions and decrease in irritation severity and burning. Seven (77.8%) patients had excellent response, 1 (11.1%) demonstrated good response, and 1 poor response. All reponders started improving within 4 - 6 weeks of therapy (16).
3.2.3. Methotrexate
Cline et al. (17) retrospectively reviewed 27 women with recalcitrant erosive vulvar LP treated with methotrexate 12.5 mg for 15.6 months. Improvement, defined as decrease in active inflammation and number of lesions at 1-month follow-up, was achieved in 70% (17). In a prospective trial by Lajevardi et al. (18), 18 patients with refractory erosive OLP received methotrexate 15 mg weekly for 12 weeks. Authors reported partial response or better in 15 (83.3%) patients, with pain reduction assessed by Visual Analogue scale (VAS) score (18). Chauhan et al. (19) compared efficacy of triamcinolone acetonide 0.1% oral paste, oral methotrexate, and combination of the 2 in moderate to severe OLP. Forty-five patients were treated for 16 weeks. Patients in the combination group had significantly better reduction in outcomes compared to the other groups (19). In a case series by Jang and Fischer (20), four women with severe longstanding erosive vulvovaginal LP received methotrexate 2.5 - 7.5 mg weekly plus topical tacrolimus and clobetasol; all cases experienced improvement in symptoms and healing of lesions within 4 - 8 weeks.
3.3. Direct Anti-T Cell Agents: Cyclosporine, Tacrolimus, and Extracorporeal Photochemotherapy
3.3.1. Cyclosporine
Two case reports demonstrated successful treatment of esophageal LP (ELP) with cyclosporine 150 mg b.i.d for 6 weeks (21, 22). Boyce et al. (21) used cyclosporine for erosive MLP with oral, genital, esophageal, and ocular involvement with secondary epiphora. After 6 weeks there was marked reduction in genital and oral ulceration, improvement of dysphagia and odynophagia, and complete epiphora resolution (21).
3.3.2. Tacrolimus
Yeo and Ormerod (23) and Chen and Sami (24) reported effective use of oral tacrolimus in OLP and genital LP. The 2 patients reported by Yeo and Ormerod (23) received 2 - 4 mg of tacrolimus b.i.d (0.05 - 0.15 mg/kg) and experienced improvement in vulvalar and oral symptoms within 6 weeks. Chen and Sami (24) treated 3 patients with tacrolimus 0.5 - 1.0 mg b.i.d; all demonstrated improvement of pain, gingival erythema, and oral lesions after 4 weeks.
3.3.3. Extracorporeal Photochemotherapy
Interest in ECP for recalcitrant LP was raised based on its clinical benefits in graft-versus-host-disease (4, 25).
Becherel et al. (26) treated 7 patients with multiresistant chronic ELP with ECP, 3 of whom also presented vulvar lesions. Mononuclear cells were extracted and soluble methoxypsoralen added to the cytapheresis product. Cells were irradiated with ultraviolet A and reinfused into patients. ECP was performed twice weekly for 3 weeks. Patients showed complete remission after 12 sessions (26). Guyot et al. (4) treated 12 patients with ECP in the same fashion. All demonstrated decrease of erosive surface; 9 (75%) achieved complete remission and 3 (25%) partial remission (4).
3.4. Other Immunomodulatory Therapies: Hydroxychloroquine, Apremilast, Thalidomide, Intravenous Immunoglobulin, and Bacillus Calmette-Guerin
3.4.1. Hydroxychloroquine
In a recent nonrandomized trial by Yeshurun et al. (27), 20 patients with erosive OLP received hydroxychloroquine sulphate 400 mg/day. Twenty-four percent achieved complete remission, 57% moderate to marked improvement, and 14% showed no improvement (27).
Results of a nonrandomized trial by Eisen (28) were similar. Nine of 10 patients treated with hydroxychloroquine 200 - 400 mg daily for 6 months showed complete healing of erosions, pain relief, and erythema reduction (28).
A retrospective analysis by Vermeer et al. (29) including 15 patients with erosive LP of the vulva and vagina treated with hydroxychloroquine between 2009 and 2020 demonstrated a 60% response rate, with almost half of patients experiencing a long-term effect.
3.4.2. Apremilast
Two case reports and two series demonstrated excellent response of OLP to apremilast 20 - 30 mg b.i.d for 4 - 24 weeks (30-33).
3.4.3. Thalidomide
In case reports by Camisa and Popovsky (34) and Petropoulou et al. (35), patients received thalidomide for 18 months for recalcitrant erosive OLP with resolution of desquamative gingivitis (34) and complete healing of erythematous and erosive lesions (35).
3.4.4. Intravenous Immunoglobulin
IVIG has been used for refractory erosive OLP in doses of 400 mg/kg/d as well as in high-doses (2 g/kg monthly), with varying responses (36, 37).
Nakashima et al. reported a case of refractory OLP who received two cycles of IVIG at 400 mg/kg/d for 5 days. Visual Analogue scale (VAS) decreased by 30% after one week of treatment and lip erosions and ulcers improved after 2 months (36). Bender et al. (37) treated three females with refractory erosive OLP with high-dose IVIG in combination with acitretin. IVIG therapy was given at 2 g/kg monthly and 0.3 - 0.5 mg/kg of acitretin for at least seven months. Clinical and subjective improvement of OLP was variable. Patient one showed no significant improvement after six monthly IVIG cycles. Patient two only received two IVIG cycles due to IVIG-induced leukopenia and demonstrated gradual significant improvement. Patient three showed a delayed, but persistent, improvement in subjective symptoms after seven IVIG cycles, however, clinical parameters did not improve to same extent (37).
3.5. Anti-Infective Agents: Doxycycline, Dapsone, Metronidazole, and Griseofulvin
3.5.1. Doxycycline
One case reported successful treatment of multiresistant ulcerative OLP with doxycycline (38) with near complete resolution of oral ulcerations and pain after 4 weeks. Topical therapies were not used, favoring effectiveness of doxycycline in OLP (38).
3.5.2. Dapsone
Beck and Brandrup (39) treated a patient with severe recalcitrant OLP for 7 months with dapsone 150 mg. Buccal lesions healed completely after 7 months and tongue erosions were reduced (39). A 9-year-old child received dapsone 1.5 - 2.5 mg/kg/d for 13 months for generalized LP with oral involvement and experienced complete clearance of lesions (40). Chopra and Kaur (41), demonstrated excellent response of MLP to dapsone in 3 months, consistent with the observations by Kumar et al. (42), who reported good response in 80% of patients with mucosal lesions.
Brewer et al. (43) presented a 30-year Mayo Clinic experience with ocular LP, through a case series of 11 patients. One patient with cicatricial conjunctivitis, keratitis, and bilateral trichiasis demonstrated considerable symptomatic and clinical improvement after dapsone and doxycycline (43).
3.5.3. Metronidazole
In a trial by Buyuk and Kavala (44) 20 patients with generalized LP and 7 with oral involvement received metronidazole 500 mg b.i.d; complete response was observed in 13 patients. In a trial by Rasi et al. (45), overall response rate to metronidazole 250 mg TID for MLP was 66.6%, with response noted after 3 months of therapy.
3.5.4. Griseofulvin
In a case series by Aufdemorte et al. (46), 3 patients with erosive OLP received griseofulvin 500 mg b.i.d, reduced to 250 mg daily after 3 months. Response was dramatic; however, response intervals varied (46). In a chart review by Massa et al. (47) involving 11 patients treated with griseofulvin for OLP, 3 obtained complete remission and 3 marked improvement. Griseofulvin 500 mg daily was used for 3 - 48 weeks (47). In trials by Matthews and Scully (48) and Bagan et al. (49), 30 patients treated with griseofulvin 500 mg b.i.d (for 3 months and 2.5 months, respectively) showed no clinical or photographic improvement, with 4 of 7 patients in Bagan’s study demonstrating worsening of the condition after treatment. Naylor (50) reported 4 patients with erosive OLP treated with griseofulvin 125 mg q.i.d for 8 weeks. No effect on pain, pigmentation changes, or disappearance of lesions was noted (50). Controversy in the literature remains, and further prospective studies are warranted to clarify efficacy of griseofulvin in OLP.
3.6. Retinoids: Acitretin/Etretinate, Alitretinoin, and Isotretinoin
3.6.1. Acitretin/Etretinate
Hersle et al. (51) conducted a randomized trial including 28 patients with severe OLP who received either etretinate 75 mg (0.75 - 1.25 mg/kg/d) or placebo daily for 2 months. Ninety-three percent of oral lesions treated with etretinate demonstrated reduction in size, infiltration and erythema compared to 5% of placebo-treated lesions (P < 0.001). Blind evaluation of clinical photos was in agreement with clinical evaluation. Sixty-six percent of patients who had improved with therapy relapsed at 3-month follow-up (51).
Zhang et al. (52) reported a 70-year-old man with multiple keratoacanthomas and who experienced marked improvement of both MLF and keratocanthomas after 1 month of acitretin 30 mg daily.
3.6.2. Isotretinoin
Woo (53) reported 2 cases of severe OLP refractory to conventional therapies that responded to oral isotretinoin rapidly and markedly. In a series by Camisa and Allen (54), 6 patients with erosive OLP received isotretinoin 10 - 60 mg daily for 8 weeks. Five patients (83%) demonstrated improvement; however, improvement was slight and relapse was noted 2 months after discontinuation (54).
3.6.3. Systemic Corticosteroids
In a retrospective review by Harewood et al. (55), 3 of 4 ELP patients responded dramatically to prednisone 40 - 60 mg for 2 - 3 weeks. All had undergone regular stricture dilatations before prednisone therapy, and 2 had worsening OLP after dilation (55). Kumar et al. (56) reported a case of extensive, atrophic OLP successfully treated with oral mini-pulse therapy with betamethasone 5 mg/day for 2 days/week for 3 weeks, then tapered until 0.5 mg/day was reached and maintained for 3 weeks. No new lesions were observed during 4-month follow-up (56). Wedgeworth et al. (57) reported on 5 patients with ELP treated with balloon dilation and intralesional triamcinolone. Each stricture was injected with 40 - 60 mg of triamcinolone in 4 quadrants (10 mg/mL aliquots), then graduated balloon dilatation was performed. All patients experienced full resolution of dysphagia for several months. This modality obviated the need for systemic immunosuppressants and associated risks (57). In a retrospective review of 100 patients with genital LP at Mayo Clinic, one-half of patients with disease remission received systemic medications, most commonly oral corticosteroids, followed by methotrexate and MMF (58).
In addition to the aforementioned treatments, there are also procedure-based interventions, including Low-Level Laser therapy, Photodynamic Therapy, plasma rich in growth factors, and submucosal autologous fat grafting, as well as nonprescription drugs including curcuminoids, total glucosides of paeony capsule and Bacillus Calmette-Guerin (BCG). These represent interesting therapeutic options for MLP. For more detailed information on these modalities, see Table 1.
| Drug | Proposed Mechanism of Action | Reference |
|---|---|---|
| Rituximab | monoclonal antibody directed against the CD20 antigen, a B-cell-specific membrane marker. B cells could have either a direct or indirect effect on CD8+ lymphocyte function, or could perhaps interfere with T-cell activation at an early stage of LP | Parmentier et al. (9) |
| Basiliximab | Basiliximab is a chimeric mouse-human monoclonal antibody against interleukin (IL)-2 receptor that inhibits T-cell activation, and therefore, may benefit patients with MLP | Rebora et al. (59) |
| Etanercept | Etanercept, a dimeric human TNF receptor fusion protein, competitively inhibits binding of TNF-α to cell surface receptors, thereby blocking TNF-mediated cellular responses. | Yarom (11) |
| Adalimumab | Adalimumab is a human recombinant immunoglobulin G1 monoclonal antibody targeted against TNF-α approved for rheumatoid arthritis, psoriatic arthritis, Crohn disease, and plaque psoriasis | Baughman et al. (13), Chao (12) |
| Mycophenolate mofetil | Mycophenolate mofetil (MMF) is a reversible, selective, and noncompetitive inhibitor of purine biosynthesis enzyme inosine monophosphate dehydrogenase, which inhibits proliferation of activated T lymphocytes and dendritic cells | Wee et al. (15), Ashack et al. (14) |
| Azathioprine | ||
| Methotrexate | Methotrexate competitively inhibits replication and function of T and B lymphocytes through competitive inhibition of dihydrofolate reductase (18). As the role of CD4 and CD8 T cells in pathogenesis of LP is well established, methotrexate is effective through down-regulation of this immunologically mediated mucosal response | Chauhan et al. (19), Jang and Fischer (20) |
| Cyclosporine | The response of MLP to cyclosporine can be explained on theoretical grounds by the T-cell-mediated pathogenesis of LP and effect of cyclosporine on this process (22). Cyclosporine inhibits production and release of IL-1 from monocytes and IL-2 from helper/inducer T lymphocytes, which play an important role in LP pathogenesis | Boyce et al. (21), Frieling et al. (3) |
| Extracorporeal photochemotherapy | Extracorporeal photochemotherapy (ECP) is hypothesized to induce apoptosis of circulating T lymphocytes. Additionally, ECP may induce an increase in plasmacytoid DC2 population with greater production of Th2 cytokine factors. | Guyot et al. (4), Zingoni et al. (25) |
| Hydroxychloroquine | Well-established immunomodulatory effects of hydroxychloroquine include reduction in regulatory T cells and inflammatory cytokines, such as IL-8, transforming growth factor-β1, and IL-10 | Yeshurun et al. (27), Zhu et al. (60) |
| Apremilast | Apremilast is a phosphodiesterase-4 inhibitor approved for moderate-to-severe plaque psoriasis and psoriatic arthritis | AbuHilal et al. (30), Bettencourt (31) |
| Thalidomide | Thalidomide has specific inhibition of TNF-α and decreases IL-12 and interferon-γ production | Petropoulou et al. (35) |
| Intravenous immunoglobulin | Intravenous immunoglobulin (IVIG) has a wide range of immunomodulating effects, including reduced T-cell proliferation and activation, and reduced release of proinflammatory cytokines and lymphokines | Nakashima et al. (36), Bender et al. (37) |
| Bacillus Calmette-Guerin-polysaccharide nucleic acid | It is hypothesized that the mechanism of action of Bacillus Calmette-Guerin-polysaccharide nucleic acid is related to its ability to induce cell-mediated immune response with an increase in serum IL-12 levels | Nasr et al. (61) |
| Dapsone | Rationale for dapsone use in LP is related to its anti-inflammatory properties through inhibition of myeloperoxidase hydrogen peroxide cytotoxic system (44). The effect of dapsone in lymphocyte-rich dermatoses, such as LP, may be similar to the mechanism proposed for neutrophilic dermatoses | Chopra and Kaur (62), Beck and Brandrup (39) |
| Metronidazole | The immunologic activity of metronidazole in LP is supported by its suppression of cell-mediated immunity and decrease in neutrophil-generated reactive oxygen species at inflammation sites | Buyuk and Kavala (44) |
| Alitretinoin | Alitretinoin (9-cis-retinoic acid) is an endogenous vitamin A derivative used for treatment of hand eczema and Kaposi sarcoma. By binding to 2 specific retinoic acid nuclear receptors, it affects cell proliferation, differentiation, and inflammation | Brehmer et al. (63), Kolios et al. (64) |
| Isotretinoin | Isotretinoin (13-cis-retinoic acid) is a vitamin A derivate primarily used for severe acne. The mechanism by which it improves MLP is not completely understood, but its anti-inflammatory and antikeratinizing effects may play a role | Giustina et al. (65) |
| Low-level laser therapy | Low-level laser therapy (LLLT) is a laser modality with biostimulating effects capable of changing cell function in a nonthermal and nondestructive manner, leading to anti-inflammatory and analgesic effects, acceleration of tissue healing, vasodilatation, and immune modulation | Agha-Hosseini et al. (66) |
| Ozone therapy | Ozone therapy is a nonmedical treatment used for OLP in dentistry, performed using an ozone generator intraorally for a few seconds twice weekly. Ozone induces immunomodulatory and antioxidant effects, similar to the biostimulatory properties of LLLT. | Kazancioglu and Erisen (67) |
| PDT | The mechanism of action of PDT in MLP may be attributed to its immunomodulatory effect. PDT-mediated oxidative stress induces apoptosis of inflammatory proliferative cells in MLP, through impairment of mitochondrial integrity and function, resulting in cell death. Additionally, IL-6 and IL-1 are up-regulated after PDT, which may also contribute to its effect | Mostafa et al. (68) |
| PRGF | Biomolecules derived from PRGF are believed to supplement growth factors and molecules, deficient at OLP lesion sites, improving cell functions and restoring cell-matrix communication | Pinas et al. (69) |
| Total glucosides of paeony | Total glucosides of paeony is a powdered substance from the roots of Paeonia lactiflora pall, a traditional Chinese herbal medication. Its main effective component is paeoniflorin, which has anti-inflammatory, immunoregulatory, and anti-oxidant effects | Zhou et al. (70) |
4. Discussion
Systemic corticosteroid therapy is reliably associated with prompt improvement of mucosal lesions, but relapse is frequent as the dose is reduced. Furthermore, oral corticosteroids are not an acceptable long-term treatment option, given well-known adverse effects.
When considering anti-T-cell agents and antimetabolities, both appear similarly efficacious in MLP; however, more data are available to support the use of antimetabolites. Among these, MMF and methotrexate represent good options for recalcitrant and refractory long-standing disease, especially when long-term management is required. They tend to be well-tolerated with manageable adverse effects.
Retinoids, such as acitretin, are of value in mild disease or as adjuvant therapy in severe disease, given their nonimmunosuppresive action; however, further studies are needed to determine ideal dosing. Based on the authors’ experience, retinoids have shown to be particularly effective in OLP. Unfortunately, their use is limited by high incidence of adverse events and their pregnancy category X determination.
Hydroxychloroquine may be an effective and safe option for erosive OLP, as demonstrated by 3 clinical trials and 1 case report (27, 28, 60, 71). It may be used in minor disease or as adjuvant in severe disease.
Apremilast may represent a valuable immunomodulatory option for MLP, with an acceptable safety profile and good tolerability in the literature (30-33). Its effectiveness in other ulcerative mucosal conditions may represent a possible emerging option in MLP.
Use of biologics in MLP has demonstrated encouraging results; however, the unavailability of alefacept and efalizumab narrow the therapeutic options in this group. Nevertheless, the positive response of MLP to these biologics warrants study of others with similar mechanisms of action. Limited data are available regarding the IL-17/IL-23 axis, which may represent an important therapeutic target in MLP. Rituximab is an appealing option, specifically for ELP.
Among procedure-based interventions, ECP might constitute a salvage option for multiresistant erosive OLP. It is relatively safe, with low infection risk; however, it is costly and time-consuming, and remission requires prolonged treatment with spaced sessions.
Although evidence is limited to 1 case report and 1 trial with mixed responses (36, 37), IVIG may be an optional target for refractory OLP. However, high costs may limit its use in recalcitrant disease.
In the antibiotic category, data are limited and reports of improvement have several cofounding factors, making these not candidates for first line therapy. Both doxycycline and metronidazole have limited supporting data for their use, and controversy exists regarding efficicacy of griseofulivin in OLP. Mode of administration is also challenging, especially with ELP where patients cannot swallow pills; oral medications need to be crushable or available in liquid form.
Management of MLP can be challenging due to limited guidelines available and lack of randomized controlled trials, with most studies demonstrating low levels of evidence. A major limitation of the present review is the descriptive nature of most MLP studies, making it difficult to compare different grading scales and results. Another limitation was the fact that Publication language was limited to English, since reviews exclusively based on English-language reports may be at higher risk of bias.
4.1. Proposal for a Treatment Algorithm
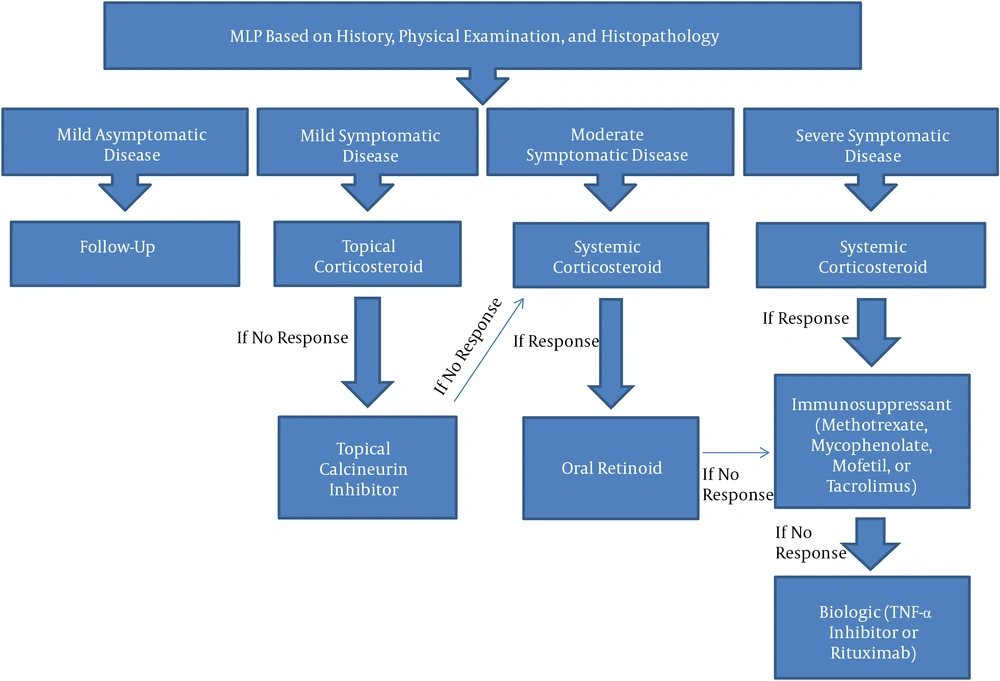
We propose clinical criteria for therapy initiation in MLP, as well as a treatment algorithm created using data from this review and our personal experience in an academic tertiary referral center.
Specific criteria for initiation of early systemic therapy in MLP include 1- severe disease;2- lack of response to conventional (topical) therapy, 3- mucous membrane involvement (e.g., eyes, ears, esophagus), and 4- involvement of organs at risk of non-function (e.g., esophagus, ocular glands, vagina). Based on our personal experience, early aggressive therapy with immunosuppressants, particularly methotrexate and tacrolimus, is recommended if more then one criteria is present. However, this statement is anecdotal and requires further studies for validation.
The authors propose a treatment algorithm for management of MLP (Figure 1). For mild and localized disease without organ compromise, topical therapies are recommended (step 1), including topical corticosteroids and topical calcineurin inhibitors alone or in combination. For severe disease nonresponsive to topical therapy or with risk of organ compromise, a stepwise approach to escalating therapy is recommended. This should start with systemic corticosteroids (step 2) used as a bridging-therapy to the next step. Step 3 comprises immunomodulatory therapies such as azathioprine, cyclosporine, methotrexate, MMF and thalidomide. Finally, step 4 utilizes biologic agents, which have been increasingly used in MLP, including rituximab, basiliximab, adalimumab, and etanercept, among others. These may be used after step 3 medications have failed or as initial systemic treatment for severe disease when step 2 therapies are not recommended (2).
5. Conclusions
In conclusion, the therapeutic algorithm proposed by the authors may be appropriately used for management of MLP. However, further larger studies including randomized clinical trials are recommended in order to confirm efficacy and therefore scientifically support this treatment algorithm.