1. Background
Acne vulgaris is a multifactorial skin disease that affects many people around the world during their lives (1). Almost 9.4% of the world’s population are affected by this skin condition (2). It is more common in young adults and has a devastating impact on the quality of life (3). According to the global burden of disease (GBD), nearly 85% of the young adults aged 12 to 15 years suffer from acne, and the disease is the tenth most common cause of disability-adjusted life year (DALY) during late adolescence in developed countries (2, 3). It can last up to the fifth decade of life, with 50.9% of the women aged 20 to 29 years and 26.3% of the women aged 40 to 49 years suffering from this skin disorder (1).
Acne vulgaris is the most common type of acne and can cause severe emotional distress and psychological disorders such as poor self-image, depression, and anxiety. In a 2019 study carried out on 165 female medical students diagnosed with acne, 66.7% were embarrassed by their skin condition, and 60% of the participants experienced disturbances in their social activities (2).
There are various forms of acne vulgaris, including comedones, pustules, papules, nodules, and cysts (1). The areas mostly affected by acne include the face, chest, back, and arms because these locations contain more sebaceous glands (4).
There are four main pathologic factors leading to acne-prone skin: the release of inflammatory mediators into the skin, follicular hyperkeratinization, follicular colonization of cutibacterium acnes, and excess sebum produced by sebaceous glands (5).
Since acne vulgaris is resulted from the hypersensitivity of skin fat glands to normal levels of androgens, genetic factors may also play important roles in acne development. According to previous studies, consuming specific medications, excessive exposure to the sunlight, use of abrasive and compressive wear, endocrine disorders, and genetic factors can be possible etiologic factors for acne (6).
A person’s lifestyle, behaviors and attitudes, include different life aspects such as dietary habits, personal hygiene, and physical activity. Among acne patients, diet and hygiene are believed to play important roles in the exacerbation of skin lesions. Many patients with acne consider poor hygiene to be the main cause of their condition, and as a result, they attempt to treat the lesion by frequent skin cleansing. Studies on this subject suggest that gentle cleansing can diminish the extent and severity of acne lesions (7, 8).
The relationship between acne and diet, especially dairy consumption and taking high-glycemic-load diets, has been studied in recent years. Among various foods, dairy products and foods with a high-glycemic index have been found to be associated with acne development (9, 10). The relationship between dairy intake and acne in adolescents and young adults has been widely studied in different countries. High-glycemic-load diets and milk-derived amino acids lead to insulin secretion and hepatic insulin-like growth factor-1 (IGF-1) synthesis (9). The plasma level of IGF-1, which stimulates follicular epithelial growth and keratinization, has been associated with the severity of acne (11, 12).
Although numerous studies have been conducted to assess the link between diet and acne formation, there is still a lack of information regarding the role of various lifestyle and dietary habits (e.g., using antioxidants, omega-3 fatty acids, vitamin A, zinc, and fiber) in acne development, necessitating further investigations on this subject (13).
2. Objectives
In this study, we aimed to assess the role of several essential lifestyle habits in the development of acne vulgaris, including water intake, vegetable consumption, physical activity, and personal hygiene. Our goal was to provide a broader perspective on the issue and to encourage more studies in this area in future.
3. Methods
This case-control study was conducted on 425 patients who attended the dermatology clinic of Qazvin University of Medical Sciences during the summer and autumn of 2019. Of these, 171 were diagnosed with mild to moderate acne vulgaris by a dermatologist, and 254 were detected with non-acne skin abnormalities. To enhance the study’s accuracy, the number of subjects in the control group (n = 254) was 1.5 times higher than that of the case group (n = 127).
The participants who aged between 15 to 35 years were included in the study. Those with polycystic ovarian syndrome (PCOs), pregnant women, as well as those taking steroids, lithium, anticonvulsants, and hormonal contraceptives were excluded (6).
This study was approved by the Ethics Committee of Qazvin University of Medical Science, and the study’s goals were clearly explained to the patients, with the assurance that their information would be kept confidential. All individuals filled out a consent form. Among those willing to cooperate, a total of 171 acne patients and 254 non-acne patients were considered as the case and control groups, respectively. The data was gathered by interviews, observations, and clinical examinations in the university’s dermatology clinic.
All patients were then asked to fill out a questionnaire containing 20 questions about demographic information and lifestyle factors. The demographic information consisted of sex, age, education level, and weight. The educational level of the participants was included to draw a better judgment on the studied population.
Lifestyle factors included dairy servings per day, fast-food meals per week, the number of fruits taken per day, vegetable portions per day, the number of glasses of water consumed per day, the number of episodes and minutes of aerobic physical activity per week, and the frequency of bathing per week. To minimize possible memory errors, the consumption of fruits, vegetables, dairy products, and water was questioned within the last two weeks. The items that were less likely to be affected by memory errors, such as physical activity, bathing frequency, and fast-food consumption, were evaluated within the last six months.
Fruits and vegetables were defined as low-energy foods rich in vitamins, minerals, and fiber. Although potatoes and cereals are sometimes defined as vegetables, in most cases, they are known as starchy foods. Green beans were also included under vegetables. Nuts, which are rich in unsaturated fats and proteins, are mostly categorized along with dried legumes under the protein category (14).
Food-frequency questionnaire (FFQ) is used to estimate the frequency of consuming specific foods by people over a determined period. Food-frequency questionnaire is now one of the two most widely used methods to estimate fruit and vegetable intake (14).
One serving of vegetables was considered as consuming one cup of raw leafy vegetables or half cup of other vegetables (either raw or cooked). One serving of fruits was considered as one medium apple, banana, orange, pear, or half cup of chopped or canned fruit, a quarter cup of dried fruit, and three-quarter cup of 100% fruit or vegetable juice (Table 1). Finally, one serving of dairy products was considered as consuming either one cup of milk or yogurt or two ounces of cheese (15).
| Vegetables | Fruits |
|---|---|
| Lettuce, cabbages; parsley, basil, coriander, chives, mint, spinach; cucumber, tomato, zucchini, chili peppers, capsicums, eggplant, okra, green beans; carrots, turnips; onions, garlic, radishes; celery | Apples, apricot, peach, nectarine, pear, plums, persimmon; citrus, kiwi, pomegranate; berries, cherries, grapes; melons, mango, banana, pineapple, figs |
The obtained data were analyzed by SPSS software version 16. The significance level was considered as P < 0.05.
4. Results
The study population consisted of 425 individuals, of whom 171 had acne vulgaris (i.e., the case group) while 254 were diagnosed with non-acne skin abnormalities (i.e., the control group). The educational level of the participants has been shown in Table 2.
| Educational Levels | Acne Group | Non-acne Group |
|---|---|---|
| High school student | 34 (19.88) | 42 (16.53) |
| High school diploma | 43 (25.14) | 77 (30.31) |
| Associate degree | 21 (12.28) | 41 (16.14) |
| Bachelor’s degree | 58 (33.91) | 66 (25.98) |
| Master’s degree | 15 (8.77) | 28 (11.02) |
a Values are expressed as No. (%).
The mean ages of the participants in the case and control groups were 21.58 ± 4.69 and 21.74 ± 5.19 years, respectively. The means of weight in the case and control groups were 64.90 ± 14.12 and 69.50 ± 12.23 kg, respectively, showing a statistically significant difference (P = 0.001) (Table 3).
| Variables | Acne Group | Non-acne Group | Total | P Value |
|---|---|---|---|---|
| Age (y) | 21.58 ± 4.69 | 21.74 ± 5.19 | 21.68 ± 4.9 | 0.7 |
| Weight (kg) | 64.9 ± 14.12 | 69.5 ± 12.23 | 67.65 ± 13 | 0.001 |
a Values are expressed as mean ± SD unless otherwise indicated.
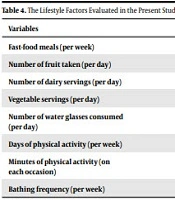
In the case group, the number of fast-food meals per week was significantly higher compared with the control group (P = 0.008). On the other hand, the number of dairy servings per day and bathing frequency were significantly higher in the non-acne group compared with the acne group (P < 0.001 and P = 0.001, respectively) (Tables 4 and 5).
| Variables | Acne Group | Non-acne Group | P Value |
|---|---|---|---|
| Fast-food meals (per week) | 1.09 ± 1.16 | 0.81 ± 0.85 | 0.008 |
| Number of fruit taken (per day) | 2.43 ± 1.85 | 2.7 ± 1.84 | 0.1 |
| Number of dairy servings (per day) | 1.17 ± 0.65 | 1.75 ± 1.31 | < 0.001 |
| Vegetable servings (per day) | 2.49 ± 1.87 | 2.78 ± 1.98 | 0.1 |
| Number of water glasses consumed (per day) | 5.64 ± 3.07 | 5.42 ± 2.32 | 0.4 |
| Days of physical activity (per week) | 3.25 ± 5.44 | 3.13 ± 2.02 | 0.8 |
| Minutes of physical activity (on each occasion) | 38.84 ± 43.11 | 38.79 ± 30.89 | 0.9 |
| Bathing frequency (per week) | 3.44 ± 1.7 | 3.99 ± 1.62 | 0.001 |
a Values are expressed as mean ± SD unless otherwise indicated.
| Variables | Beta Coefficient | Standard Error | P Value | Odd Ratio |
|---|---|---|---|---|
| Fast-food meals (per week) | -0.384 | 0.143 | 0.008 | 0.681 |
| Number of dairy servings (per day) | 0.700 | 0.174 | 0.00 | 2.013 |
| Bathing frequency (per week) | 0.137 | 0.081 | 0.001 | 1.146 |
| Weight | 0.038 | 0.011 | 0.001 | 1.039 |
Vegetable and fruit intake was higher in the non-acne group than in the acne group, but the difference was not statistically significant (P = 0.1) (Table 4). The number of glasses of water consumed per day was not significantly different between the two groups (P = 0.4).
The episodes and minutes of physical activity per week were higher in the case than in the control group, but the difference was not statistically significant (P = 0.8 and P = 0.9, respectively) (Table 4).
5. Discussion
Demographic findings such as age and weight were evaluated in the current study. The mean ages of the participants in the acne and non-acne groups were 21.58 and 21.74 years, respectively. In a 2018 study, out of 1,167 acne patients, 454 (41.3%) were adults, and 713 were adolescents (58.7%) (16). In another study, acne was reported to be more prevalent among teenagers, but 45% of the women aged 21 to 30 years also suffered from this skin disease (17). In a 2018 cross-sectional study on adolescents and young adults in seven European countries, the highest prevalence of acne was reported between the age of 15 to 17 years (18). In the current study; however, only the patients who aged 15 to 35 years were included.
In the present study, the means of weight in the case and control groups were 64.90 ± 14.12 and 69.50 ± 12.23 kg, respectively, indicating a significant difference (P = 0.001). In another study in 2018, most acne patients had a normal body mass index, and there was no significant relationship between weight and acne severity (19). In 2019, a study conducted on 600,404 adolescents reported that as weight increased, the incidence of acne decreased gradually (20).
In this study, the participants in the acne group consumed significantly more fast foods than those in the non-acne group (P = 0.008). Another study on the relationship between diet and acne vulgaris found that patients with a western diet, which includes more junk foods, experienced more severe acne (21). In another study, it was reported that fast-food consumption could flare up acne lesions (22). In a study on the relationship between acne and dietary habits, it was found that frequent fat intake and use of sausages and burgers were associated with an increased incidence of acne lesions (23).
In our study, dairy consumption was significantly higher in the non-acne group than in the acne group (P < 0.001). This was inconsistent with the findings of most studies. In a 2018 systematic review and meta-analysis on 78,529 individuals, consuming any dairy product was associated with an increased risk of acne (9). In another similar study, no significant difference was found in total dairy intake comparing acne patients and non-acne counterparts (24).
The amount of vegetable and fruit intake was higher in the non-acne group than in the acne group, but the difference was not statistically significant (P = 0.1). In another study, it was reported that low weekly consumption of fruits or vegetables was associated with more severe acne (25). Another study reported a decrease in acne severity following the consumption of a diet rich in vegetables and fruits (21).
The number of episodes and minutes of physical activity were both higher in the non-acne group compared with the acne group, but the differences were not statistically significant. In a similar study in 2019, exercise was reported to be significantly more common among non-acne patients compared with acne patients (26). In another study on the effect of exercise-induced sweating on facial sebum, it was observed that sebum production increased while skin pH decreased during exercises, raising the risk of acne (27).
Due to the lack of information on the impact of water consumption on acne formation, we evaluated water intake in both case and control groups. The mean number of water glasses consumed per day was higher in the acne group (5.64) compared with the non-acne group (5.42); however, the difference was not statistically significant (P = 0.4).
Bathing frequency in the non-acne group was significantly higher compared with the acne group (0.001). No previous study was found on the link between bathing frequency and acne development, but in a clinical trial on 29 women, investigating the effect of facial cleansing on acne formation, lesions significantly improved after the intervention (28). Most other studies have evaluated the efficacy of a particular product, which was not of interest to our study.
5.1. Conclusion
Most previous studies have focused on the factors exacerbating acne formation in patients. Since this work was a comparison between acne patients and non-acne counterparts, our results may be indicative of whether main lifestyle aspects including dietary habits, exercise, and bathing frequency can have important roles in the incidence of acne. There were no significant differences in most of the studied lifestyle factors between the two groups. Therefore, it is recommended to divulge the possible roles of other underlying factors such as genetic and hormonal determinants in the risk of acne vulgaris.