1. Introduction
Linear immunoglobulin A (IgA) dermatosis is a rare autoimmune vesiculobullous disease characterized by homogeneous linear IgA deposits on the basement membrane of the epidermis, and it can affect skin with/without mucosa (1, 2). This disease is often referred to as linear IgA bullous dermatosis (LABD) or linear IgA disease (3), and it can be idiopathic or drug-induced (4, 5). Drug-induced linear IgA dermatosis is generally the result of systemic vancomycin use, (1) but several case reports suggested that various other drugs can also cause linear IgA dermatosis such as antibiotics, analgesics, antihypertensives, antiepileptics, or immunosuppressants (4-6). The incidence of liner IgA dermatosis is very rare (0.2 - 2.3 cases per one million population per year). Drug-induced linear IgA dermatosis cases were reported to be 37.5% in adults (5). According to the patient data at RSUP Dr. Mohammad Hosein (RSMH) Hospital in Palembang, Indonesia, there were two patients with idiopathic linear IgA dermatosis and one suspected drug-induced case in the period of 2014 - 2020.
The pathogenesis of drug-induced linear IgA dermatosis is not fully known yet. In drug-induced linear IgA dermatosis, IgA antibodies against LAD285 and BP180 are found (7). The related drugs can induce an autoimmune response through a cross-reaction mechanism with the epitope target, ie, changing the arrangement of the epitopes or bringing previously hidden antigens to the immune system (8).
The general clinical features resemble idiopathic linear IgA dermatosis in vesiculobullous eruptions, erythema plaques, target or target-like lesions, or string of pearls. Predilection of the superior and inferior limbs, palms and feet, trunk, buttocks, and face or neck, and in some cases mucosal involvement are reported. Subjective symptoms include pruritus and burning (5). Drug-induced linear IgA dermatosis usually remits spontaneously after the offending drug is discontinued (5), but in some cases, additional therapy such as topical and systemic corticosteroids, dapsone, sulfonamides, colchicine, or intravenous immunoglobulin (IVIG) may be required (9).
In this study, we reported a 17-year-old man with anti-TB drug-induced linear IgA dermatosis to study its clinical, histopathological, and immunofluorescent features.
2. Case Presentation
A 17-year-old male referred to our center with complaints about red spots and tense blisters all over his body, accompanied with itching and burning sensation since one day ago. There were several coin-size red spots in the right back with itchy and burning sensation since four days ago. The red spots were spread almost to the entire back, chest, arms, legs, oral cavity, nose, head, both palms and feet, and genitals one day ago; some lesions were changing to a group of tense blisters with burning sensation. The itching was getting worse until it was difficult to sleep. Swallowing pain was present. The patient was undergoing pulmonary TB treatment consisting of a combination of rifampicin, isoniazid, pyrazinamide, and ethambutol since one week ago. The patient did not seek treatment but only continued pulmonary TB drugs. There was no history of alergy in the patient and his family members. There was no history of taking other drugs in the last three months. No family member had similar complaint.
Vital signs were within normal limits, and he was underweight (BMI: 13.67). Physical examination revealed hyperemic pharynx, left lung vesicular breath sounds (VBS) decreased, and rhonchi appeared. On physical examination, widespread plaques erythema were seen over the patient's facial, trunk, extremity, genitalia, manus, and pedis, with clusters of the vesicles having a 'string of pearls' appearance visible on the borders. There were some erosions and tense bullae in oral and nasal cavity. The region of the nasal cavity contained vesicles, skin-colored base, and scattered multiple vesicles less than 1 cm in size. The oral region contained erosions-excoriation, irregular, tense bullae, skin-colored base erythema, multiple, millier-lenticular, grouped, and scattered. Spreading of lesions was 0.5% in head, 5.5% in trunk, 3% in extremity, 1% in genitalia, and total BSA was 10%. Asboe-Hansen examination was performed with negative result, meaning no acantholysis.
Several additional examinations were performed, such as microscopic examination using gram staining with erosion swab specimens in the trunk region. The results were epithelial cells 3 - 5 LPB, PMN 1 - 5 LPB cells, coccus, and no Gram-positive or Gram-negative bacilli were found. Chest X-ray showed left lung tuberculosis (TB) with pleural effusion. The patient's diagnosis was established as suspected anti-TB drug-induced linear IgA dermatosis with suspected anti-TB drugs + pulmonary TB new case. The topical management of bullae aspiration, compresses of 0.9% NaCl solution for 10 minutes on blisters every 12 hours, and intrasite®gel every 12 hours after compressing, systemic treatment, and cetirizine 10 mg tablets orally every 24 hours were prescribed.
at day 3, the previous lesions were not repaired, and there were new lesions. Other additional examinations were performed. The previous therapy was continued, and additional topical therapy was given; desoximetasone cream 0.25% every 12 hours on the rash and systemic therapy with methyl prednisolone 24 mg tablets 8 mg (2-1-0) orally for seven days and 24 mg were prescribed.
at day 11, there was improvement in the old lesions, but new blisters were seen on the chest and arms, and itching got worse. The laboratory results showed increasing value of LED 93 (N: <30) mm/hour, neutrophils 83 (N: 50 - 70%), and IgE 1,393.6 (N: <100) IU/L and decreasing value of lymphocytes 8 (N: 20 - 40%). The histopathological biopsy results were derived from the skin biopsy of the right arm, and the epidermis was covered with keratinized squamous-complex epithelium. It appears that the subepidermal bullae contain a lot of neutrophil cells, fibrin, dilated blood vessels, and there is perivascular infiltration in the form of neutrophils. There were no signs of malignancy in this preparation. The conclusion was IgA dermatosis with dermatitis herpetiformis as a differential diagnosis. The results of direct immunofluorescence (DIF) examination of skin preparations showed deposits of IgA and fibrinogen in the dermoepidermal junction, with a linear pattern and weak intensity. There were no IgG, IgM, and C1q deposits on the preparation. Our results showed that immunofluorescence profile can support a linear IgA dermatosis.
The diagnosis was anti-TB drug-induced linear IgA dermatosis with suspected anti-TB drug + pulmonary TB new case. Previous therapy was continued. Additional systemic therapy was performed by prescribing 24 mg methyl prednisolone tablet 8 mg (2-1-0) for three days followed by 16 mg dosage form 16 mg (1-0-0) for 7 days orally, cetirizine tablet 10 mg every 24 hours (morning) orally, and 4 mg tablet CTM every 12 hours (day and night) orally. Desensitizing the anti-TB drugs started with isoniazid 50 mg on the first day, then new vesicles appeared, isonazid stopped. Re-desensitization started by rifampin 75 mg, and there were no new lesions, followed by pyrazinamide 250 mg and ethambutol 100 mg; isoniazid was not re-administered with no new lesions as a result.
When the patient was referred on day 21, the old lesions had improved, and the itchy sensation had been reduced. New lesions were absent. Previous therapy was continued. Additional topical therapy was given; cream 10% was applied to dry skin every 12 hours, and 12 mg methylprednisolone tablet 8 mg (2½-0-0) was systemically given for seven days orally.
at day 28, there were no new lesions, and the old lesions were improved; and the patient’s body weight increased (3 kg). Previous therapy was continued. Additional systemic therapy was given, including 8 mg (1-0-0) methylprednisolone tablets for seven days orally followed by 8 mg (1-0-0) doses alternate for seven days; then treatment was stopped.
3. Discussion
Linear IgA dermatosis is a chronic vesicobulosic autoimmune disease characterized by subepidermal bullae due to homogeneous linear IgA deposits against antigens on the basement membrane (1, 2, 10, 11). This disease is often referred to as linear IgA bullous dermatosis (LABD) or linear IgA disease (3).
Linear IgA dermatosis can occur at any age. Meanwhile, drug-induced cases are more prevalent among older and male patients. The incidence of this disease is around 0.2 to 2.3 per one million people per year (2, 3, 5, 10). Lings and Bygun compared the idiopathic and drug-induced cases, and it was found that the mean age of the onset of drug-induced linear IgA dermatosis was 66.5 years, whereas in idiopathic patients the mean age was 51 years with a male to female ratio of 1.0: 0.5 (12). In our case, the disease was found in a male patient aged 17 years.
Although 50% of cases are induced by vancomycin, other antibiotics, including penicillins, cephalosporins, and sulfonamides, can trigger the formation of IgA antibodies (5). In a study by Tsai, four (25%) patients reported a history of drug ingestion shortly before the onset of the linear IgA dermatosis, including rifampin, vancomycin, gemcitabine, and griseovulvin (11). In this case, erythema plaques were found, some of which became vesicles and bullae with pruritus appearing on the third day after taking anti-TB drugs routinely in the morning.
The mechanism of the drug able to stimulate a person’s immune system to produce IgA antibodies against the basement membrane in linear IgA dermatosis is not clear yet (5). Some drugs can act as hapten, dermal protein/epidermal complex, and provide autoimmune response (4). Circulation of IgA antibodies to 97-kDa or 120-kDa bullous pemphigoid antigen 2 (BPAG2) extracellular domain BP180 in the basement membrane zone (BMZ) is said to be the main underlying cause (2, 11, 13).
Drug-specific T cells play an important role by releasing T helper 2 (Th2) cytokines such as interleukin-4 (IL-4), IL-5, IL-6, IL-10, and transforming growth factor-β (TGF β), which stimulate production of IgA antibodies (5, 11). In a study by Yawalkar et al. (14) there was a significant increase in levels of IL-5 and interferon-IF (IFN-γ) in the lymphocyte transformation test (LTT) supernatant culture. CD8+ cytotoxic T lymphocytes are believed to have an important role in triggering the initial formation of autoantigens (5). The main antigen targets in idiopathic linear IgA dermatosis are LAD285, BP180, and BP230. Meanwhile, in drug-induced cases, IgA antibodies against LAD285 and BP180 were also found. Related drugs can cause an autoimmune response through a cross-reaction mechanism with the target epitope, such as changing the arrangement of the epitope or bringing previously hidden antigens to the immune system. Collagen type VII (COL7) was identified as the primary autoantigen IgA antibody (1). Pulmonary TB infection in patients can also be suspected as a predisposing factor to trigger the anti-TB drug-induced linear IgA dermatosis. The resolution of vesicles and bullae gradually occurs after the anti-TB drugs have temporarily stopped desensitization. This proves that anti-TB drugs are more likely to cause linear IgA dermatosis than the possibility of pulmonary infection.
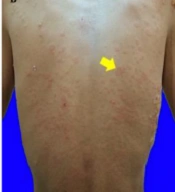
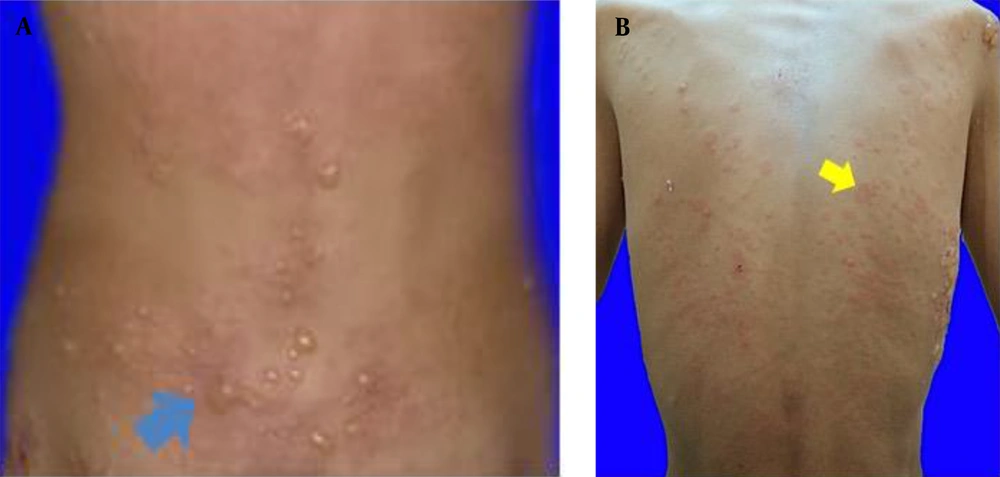
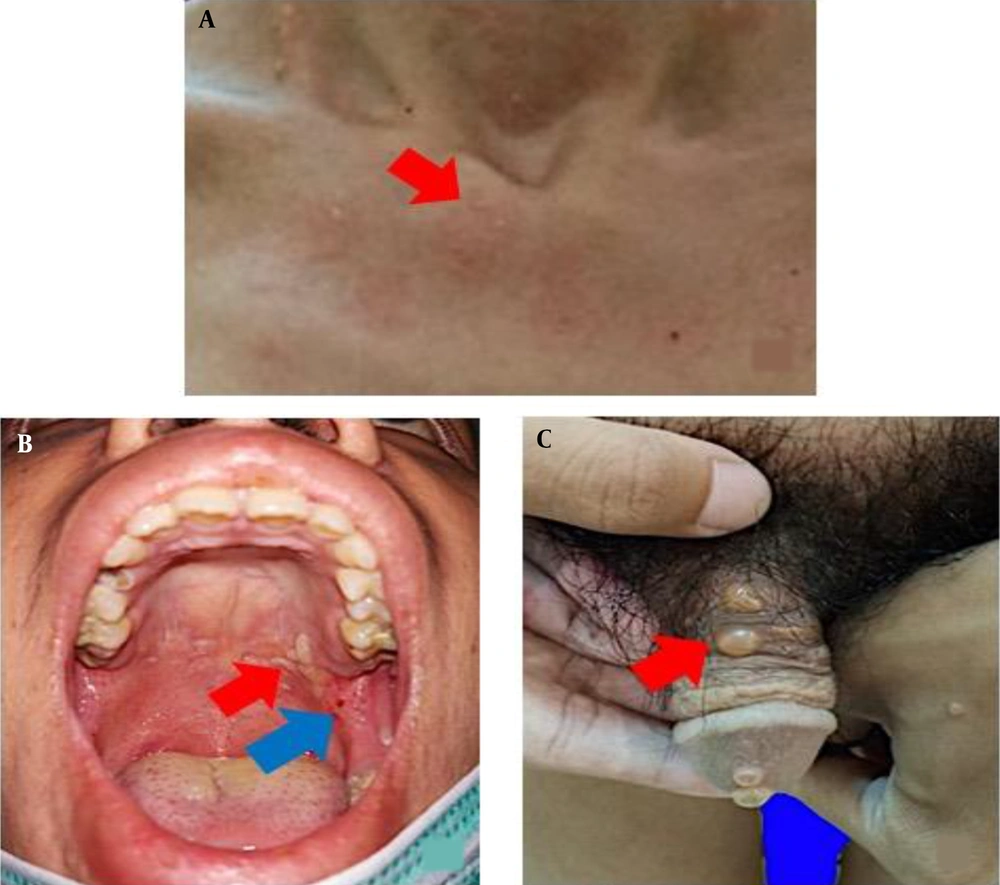
The clinical features of idiopathic and drug-induced linear IgA dermatosis vary. The drug-induced linear IgA dermatosis show vesiculobullous eruptions, erythema plaques, target lesions, or target-like lesions/"string of pearls" is resembling erythema multiforme (EM) lesions, clustered tense bullae "clusters of jewels" is resembling bullous pemphigoid (PB) or papulo-vesicles and pruritus is resembling dermatitis herpetiformis (DH) (1, 5, 6, 11). Chanal's study found a picture of erythema plaque lesions in 9 (75%) patients, 6 (50%) patients had targeted lesions or similar to target lesions (50%), and 4 (33%) patients had string of pearls (15). Annular or clustered papules, vesicles and bullae are present in 50% of cases. Predilection is common in the superior and inferior limbs, including the palms of the hands and feet, and the trunk, buttocks, and face/neck (5). Tsai et al. (11) reported trunk involvement in 76% of patients, limbs in 62%, and perineum in 12%. Mucosal involvement was found in the range of 40% in drug-induced cases but higher (80%) in idiopathic cases (1, 4). The highest rates of mucosa involvement are oral (15.8%), genital (7.8%), conjunctival (5.2%), and nasal (2.6%) (16). The lesions of drug-induced linear IgA dermatosis resemble PB and DH, in the form of vesicle-bullous erythema most basic normal skin, clear contents, some groups to form "cluster of jewel" (Figure 1A). In this case, there were also erythema-normal skin base vesicles, clear contents, some of which clustered to form a “cluster of jewel” (Figure 1A) (11). Other skin lesions are erythema plaques that are annular in shape (Figure 1B) (1), and partly there are vesicles on top of the plaque forming a circle like a "string of pearls" (Figure 2A) (11). The case report of Joseph et al. stated that there were lesions in the oral cavity and a burning sensation that made swallowing difficult (17). In the case of erosions and bullae in the oral cavity as well as erosions that make it difficult to swallow (Figure 2B), Nasr et al. reported a patient with drug-induced linear IgA dermatosis with tense bullae in the genitals (18). In this case, there was also a tense bullous vesicle with a partially erythematous normal skin base (Figure 2C).
There was no difference in the histopathological features of drug-induced and idiopathic linear IgA dermatosis, which was a subepidermal cleft that differentiates it from drug-induced pemphigus. Minimal keratinocyte necrosis and predominantly neutrophil infiltrates can distinguish it from fixed drug eruption bullous (FDE), bullous EM, SSJ, and NET, which also reveal subepidermal clefts. Subepidermal cleft with predominantly eosinophil infiltration may rule out the differential diagnosis of drug-induced PB. Histopathological examination has not been able to distinguish between DH and linear IgA dermatosis; so, immunofluorescence examination is needed (2, 6). In this case, a biopsy was performed in the left antebrachii vesicle, and the results showed a subepidermal cleft with neutrophil inflammatory cell infiltration. DIF examination in drug-induced linear IgA dermatosis found 13 - 30% linear deposits of IgA antibody in the BMZ, circulating IgA was found to be higher, and IgG was not found (1, 2, 4, 15). There was only one case of IgG deposit in drug-induced linear IgA dermatosis, whereas in idiopathic IgG was found in BMZ in 30% of cases. This may rule out the causes of linear dermatosis IgA idiopathic, as well as drug-induced DH and PB. DIF in DH found granular IgA deposits in the papillae dermis, whereas drug-induced PB would predominantly be linear deposits of IgG and C3 along the BMZ (6). The study by Yamagami on DIF examination found linear IgA deposits along the BMZ in 30 (78.9%) patients (10). In the case of a skin biopsy performed on peri-lesion in the left, antebrachii, IgA deposits were found in the dermoepidermal junction, linear pattern, and weak intensity. There were no deposits of IgG, IgM, and C1q.
The onset of clinical manifestations in drug-induced linear IgA dermatosis ranges from one day to 26 months (5). The lesions’ resolution of linear drug-induced IgA dermatosis may occur within 2 - 7 weeks after drug discontinuation. In patients, skin lesions found on the third day after consuming anti-TB drugs improved gradually within one week after the anti-TB drugs was temporarily stopped for one week and got better after anti-TB drugs desensitization. There are no studies or case reports stating that anti-TB drugs can cause linear IgA dermatosis. But some literature showed that the antibiotics can trigger linear IgA dermatosis. The antibiotics ciprofloxacin and vancomycin have been reported to cause linear IgA dermatosis (6). In a retrospective study conducted in 2019, Lammer expressed that rifampin can trigger linear IgA dermatosis (5).
Drug-induced linear IgA dermatosis is usually characterized by spontaneous remission after the suspected drug is discontinued (1, 5). Fortuna et al. studied drug-induced linear IgA dermatosis and suggested that the majority (50%) of cases still needed additional therapy even after the causative drug was discontinued. The additional therapy can avoid the spread of the disease due to the immunological signals generated by the self-maintaining immune response (9). Dapsone is the first-line therapy for linear IgA dermatosis. Alternative therapy is sulfapyridine, corticosteroids, colchicine, tetracyclines, and nicotinamide (1, 5). In drug-induced cases, methyl prednisolone 0.5 - 1 mg/kg/day was used as initial therapy; also, stopping the previous treatment responded well (11). A 2015 case report by Pinard showed that rituximab can be administered for linear of IgA dermatosis recalcitrant to dapsone, systemic steroids or other immunosuppressive agents (13). If the drug was suspected as the cause, the anti-TB drugs was temporarily stopped for one week. Patients were given 24 mg of methyl prednisolone, 10 mg of cetirizine, 0.25% desoximetasone cream, and treatment of skin lesions (bullae aspiration). After that, the anti-TB drugs were given one by one starting from isoniazid, rifampin, pyrazinamide, and ethambutol. The use of the drug started sequentially from isoniazid, rifampin, pyrazinamide, and ethambutol. Each drug was administered in a gradually increased dose over three days starting from a small to large dose (19).
Well-tolerated drugs are then given immediately at the full dose followed by subsequent administration of the drug starting at a small dose and the same procedure. When there are no skin abnormalities, one more drug is added. If a skin reaction appears after giving certain anti-TB drugs, this indicates that the anti-TB drugs given are the cause of skin reactions. After the anti-TB drugs are known to cause the skin reaction, treatment can be continued without the anti-TB drugs (19). The patient was desensitized by anti-TB drugs by the Lung Department on the first day of using isoniazid 50 mg; if there were no new lesions, rifampicin 75 mg was used on the 2nd day, pyrazinamide 250 mg on the 3rd day, and ethambutol 100 mg on the 4th day. The patient complained of developing new blisters on the arm after taking 50 mg of isoniazid. Anti-TB drugs desensitization was performed again in the form of rifampin 400 mg, pyrazinamide 1000 mg, and ethambutol 750 mg. There were no new blisters, and the old lesions improved. A possible drug causing a skin reaction is isoniazid. Methyl prednisolone was tapered off slowly and was discontinued after 42 days of treatment. After the methyl prednisolone was discontinued, no new skin lesions were found. Observations were made for up to six months to determine recurrences (4). Cases of drug-induced linear IgA dermatosis usually provide a good prognosis characterized by spontaneous remission after the suspected drug is discontinued (11). In this case, remission occurred six weeks after therapy, oral steroids were tapered off slowly and discontinued on treatment day 42. After the oral steroids were discontinued, there were no new lesions. Observations were made for up to six months to determine recurrences.