1. Background
Atopic dermatitis (AD) is a chronic and relapsing inflammatory skin disease with more prevalence in children less than five years old (1). In one study in Kerman by Farajzadeh et al., the prevalence of AD was estimated at 9.1% (2). Genetic defects in epidermal structures and impaired skin barriers may lead to dry and irritable skin in these patients. Severe itching and eczematous lesions are key features of the disease. The acute phase of AD is defined by erythema, swelling, bulla, and vesicle formation, while the chronic phase is characterized by scaling and marked skin thickness (1-3).
Former studies revealed that AD children have a lower quality of life (QoL) than those with other chronic diseases such as diabetes mellitus, epilepsy, renal disease, acne, psoriasis, and vitiligo (4). Severe pruritus in patients with AD can result in anxiety, sleepiness, and low concentration during daytime and negative effects on school performance (5, 6). Moreover, various factors such as air pollution, house dust mite, and smoking can exacerbate or trigger the disease course (7, 8). Changes in lifestyle including hobbies, physical activities, dressing, and eating habits can interfere with the inter-personal and social relationships (9).
Currently, there are different questionnaires to evaluate QoL in children with chronic disease (10-12). The English version of the Children’s Dermatology Life Quality Index (CDLQI) questionnaire exclusively evaluates QoL in children aged 4 to 16 years old. Atopic dermatitis is the most common skin disease evaluated by this questionnaire. The validity and reliability of different language versions of the questionnaire have already been evaluated (13).
2. Objectives
In this study, we decided to assess the validity and reliability of the Persian version of QOL in children with AD.
3. Methods
The CDLQL questionnaire contains 10 questions considering physical symptoms, mood changes, disturbance in educational, social, physical, and leisure activities, treatment problems, as well as adjustment in sleeping, bathing, and dressing habits in the recent week in children aged 4 to 16 years. Each question has four choices, including not at all, a little, a lot, and very much. The score of each question varies from 0 to 3. The final score is calculated as the sum of all scores that varies from 0 to 30. A higher score indicates the more influence of the disease on the QoL of children.
To create the Persian version of CDLQI, we got permission from the original authors. Two independent bilingual natives, whose first language was Persian, translated the questionnaire into Persian. Next, after approval of the first Persian translation of the questionnaire, it was back-translated to English by two additional bilingual English translators. This process continued in agreement with national translation rules until obtaining a final proof by original authors. Then, 10 children with AD were assessed to confirm the comprehensibility of the questionnaire (14).
Finally, after obtaining written consent from parents and children older than 12 years, we assessed the validity and reliability of the Persian version of the questionnaire. We surveyed from September 2015 to June 2016 in Afzalipour hospital in Kerman University of Medical Sciences. The inclusion criteria were AD children aged 4 to 16 years capable of reading and understanding the Persian language. We excluded children affected by other diseases with a negative impact on QoL. Patient characteristics and demographic data, including age, sex, family history of atopy, and severity of the disease, were collected based on SCORAD along with the socio-economic and educational condition of the family. Then, the Persian version of the CDLQ questionnaire was filled out by children with AD.
3.1. Statistical Analysis
We used mean, median, and standard deviation for descriptive analysis. Cronbach’s alpha and inter-item correlation were calculated for assessing the reliability of the questionnaire. The validity of the questionnaire was estimated by the scree plot and Kaiser criterion. We used ANOVA and independent t test to compare the scores based on demographic features. The Pearson correlation test was applied for assessing internal consistency between variables. We used SPSS 18 to analyze the data.
4. Results
One hundred AD children with a mean age of 8.31 ± 3.02 years (range 5 - 15) participated in the study. Fifty-two percent of the patients were males. A family history of atopy was reported in 41% of the participants.
4.1. CDLQI
The mean score of CDLQI was 9.44 ± 5.47 (minimum = 11, maximum = 22). The highest and lowest scores were related to questions number 1 (itching) and question number 6 (sports activities) (Table 1). Moreover, none of the participants achieved the lowest (floor effect) or the highest scores (ceiling effect) from the questionnaire. The level of the impact of QoL on patients was categorized into five groups, including no effect (0 - 1 scores), small effect (2 - 6 scores), moderate effect (7 - 12 scores), large effect (13 - 18 scores), and very large effect (19 - 30 scores). As Table 2 shows, AD moderately influenced QoL in 38% of the participants.
| Question Number | Subjects | Mean ± SD | Percent of Response Rate to Answers | |||
|---|---|---|---|---|---|---|
| Very Much | A Lot | A Little | Not at All | |||
| 1 | Itching and scratching | 1.84 ± 0.82 | 23 | 42 | 31 | 4 |
| 2 | Child mood | 1.49 ± 0.91 | 11 | 45 | 26 | 18 |
| 3 | Inter-personal relationship | 0.74 ± 0.74 | 1 | 15 | 41 | 43 |
| 4 | Dressing problems | 0.92 ± 0.86 | 4 | 21 | 38 | 37 |
| 5 | Disturbance in hobbies | 0.59 ± 0.69 | 0 | 12 | 35 | 53 |
| 6 | Disturbance in sport activities | 0.40 ± 0.76 | 7 | 21 | 71 | 9 |
| 7 | Disturbance in educational activities | 0.55 ± 0.77 | 4 | 5 | 33 | 58 |
| 8 | Disturbance in social activities | 0.51 ± 0.74 | 3 | 6 | 30 | 61 |
| 9 | Sleeping problems | 1.16 ± 0.81 | 4 | 30 | 44 | 22 |
| 10 | Treatment problems | 1.24 ± 0.79 | 2 | 40 | 38 | 20 |
Mean Scores and Percentage of Response Rates to Answers in CDLQ Questionnaire
| Percentage of Effects on QoL | Prevalence (%) |
|---|---|
| None | 6 |
| Small | 29 |
| Moderate | 38 |
| Large | 18 |
| Very large | 9 |
Percentage of Impact of Disease on Quality of Life of the Patients
4.2. SCORAD
The mean number of SCORAD was 31.97 ± 13.48 (minimum = 11.10, maximum = 57.40). The severity of the disease, according to SCORAD, was classified as mild (less than 25), moderate (25 - 50), and severe (more than 50). The severity of AD was severe, moderate, and mild in 11, 53, and 36% of the cases, respectively.
4.3. Reliability
Cronbach’s alpha was estimated at 0.87 that is indicative of good reliability. Moreover, we evaluated internal consistency between the items, and the inter-item correlation coefficient ranged from 0.24 to 0.69 (Table 3). Therefore, all of the questions had good internal consistency and nearly were higher than 0.3 (lowest acceptable range). There was no increase in Cronbach’s alpha if any of the items was deleted (Table 4). Table 5 shows the factor loading of each question.
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | 1.000 | |||||||||
| Q2 | 0.692 | 1.000 | ||||||||
| Q3 | 0.351 | 0.570 | 1.000 | |||||||
| Q4 | 0.402 | 0.510 | 0.448 | 1.000 | ||||||
| Q5 | 0.282 | 0.474 | 0.585 | 0.597 | 1.000 | |||||
| Q6 | 0.307 | 0.408 | 0.411 | 0.368 | 0.364 | 1.000 | ||||
| Q7 | 0.342 | 0.356 | 0.476 | 0.290 | 0.515 | 0.461 | 1.000 | |||
| Q8 | 0.310 | 0.398 | 0.510 | 0.359 | 0.500 | 0.345 | 0.472 | 1.000 | ||
| Q9 | 0.422 | 0.475 | 0.512 | 0.282 | 0.341 | 0.281 | 0.418 | 0.511 | 1.000 | |
| Q10 | 0.421 | 0.362 | 0.335 | 0.404 | 0.243 | 0.251 | 0.321 | 0.366 | 0.508 | 1.000 |
Inter-item Correlation Coefficients Between Questions of CDLQ Questionnaire
| Question Number | Corrected Item-Total Correlation | Cronbach's Alpha if Item Deleted |
|---|---|---|
| Q1 | 0.576 | 0.864 |
| Q2 | 0.696 | 0.854 |
| Q3 | 0.681 | 0.856 |
| Q4 | 0.586 | 0.863 |
| Q5 | 0.629 | 0.861 |
| Q6 | 0.507 | 0.869 |
| Q7 | 0.578 | 0.864 |
| Q8 | 0.602 | 0.862 |
| Q9 | 0.602 | 0.862 |
| Q10 | 0.514 | 0.869 |
Cronbach’s Alpha of Each Question and Evaluation of Reliability of the Questionnaire
| Question Number | Factor Loading |
|---|---|
| Q2 | 0.773 |
| Q3 | 0.769 |
| Q5 | 0.723 |
| Q8 | 0.697 |
| Q9 | 0.692 |
| Q4 | 0.679 |
| Q7 | 0.674 |
| Q1 | 0.657 |
| Q6 | 0.600 |
| Q10 | 0.599 |
Factor Loading of Each Question
4.4. Validity
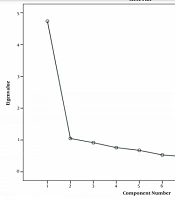
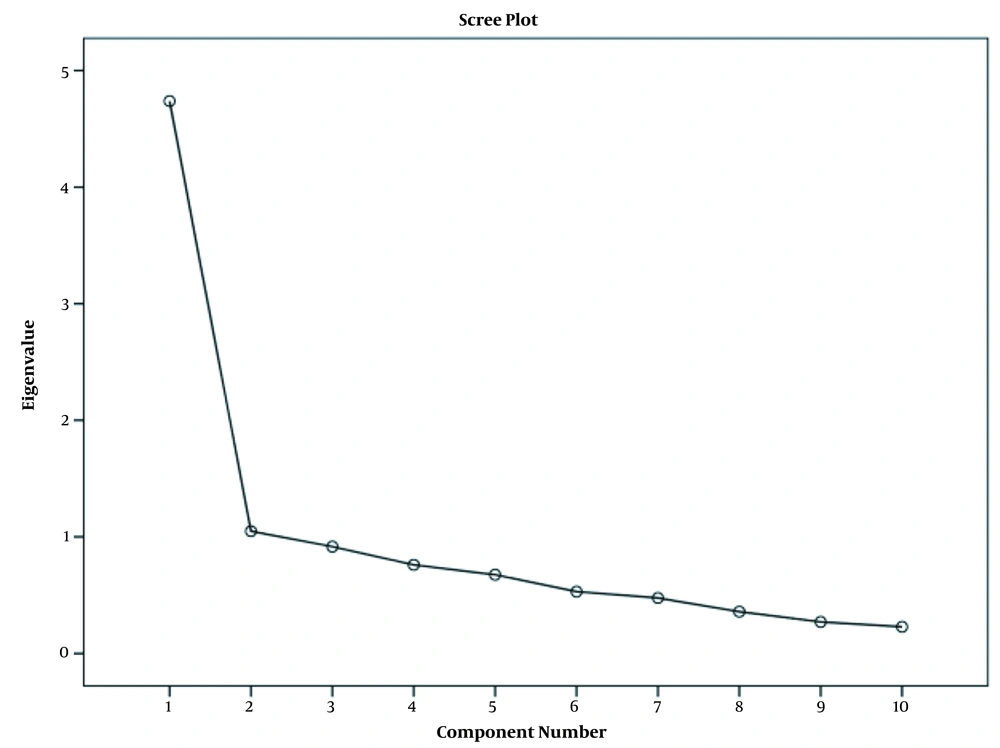
We used the scree plot and Kaiser criterion to calculate the validity, representing one-factor structure and describing 47.4% of the variance (Figure 1).
4.5. QOL and Demographic Features Correlation
We evaluated the relationships between QoL and demographic features, such as age and sex of the participants, family history of atopy, socioeconomic and educational level of parents, which were not statistically significant (Table 6). There was a strong and positive correlation between SCORAD and QoL score (P < 0.0001, r = 0.70). Consequently, children with higher SCORAD (more severe disease) had lower QoL.
| Variables | Mean ± SD | Number | P Value |
|---|---|---|---|
| Sex | 0.93 | ||
| Female | 9.39 ± 5.35 | 48 | |
| Male | 9.48 ± 5.63 | 52 | |
| Family history of atopy | 0.68 | ||
| Yes | 9.58 ± 6.66 | 41 | |
| No | 9.13 ± 5.20 | 58 | |
| Parents’ educational level | 0.87 | ||
| High school diploma or less | 9.02 ± 5.16 | 36 | |
| Bachelor of Science | 9.29 ± 6.20 | 24 | |
| Master of Science | 10.19 ± 5.83 | 26 | |
| PhD | 9.35 ± 4.61 | 14 | |
| Parents’ economic level | 0.90 | ||
| Weak | 9.90 ± 5.43 | 22 | |
| Moderate | 9.29 ± 5.26 | 54 | |
| Good | 9.33 ± 6.15 | 24 |
Evaluation of Correlation Between Demographic Features of the Patients and Quality of Life
5. Discussion
Chronic skin diseases such as AD affect the physical appearance and lifestyle of the patients and may lead to learning difficulties, behavioral problems, and cognitive impairment, and have negative impacts on the QoL of the patients (10, 11, 15). Hence, it is always necessary to develop a standard questionnaire to assess the QoL of the patients (16-18). The CDLQI questionnaire specifically evaluates the QoL of children with dermatitis aged 4 to 16 years old (19, 20). The current study revealed a mean score of 9.44 ± 5.47 that was in agreement with the results of a meta-analysis by Olsen et al. (7.1 - 9.8), representing the moderate effect of AD on the QoL of these patients (4). Currently, the validity and reliability of this questionnaire are evaluated in different countries with reported Cronbach’s alpha values from 0.82 to 0.92 (13, 21). Cronbach’s alpha in our study was 0.87, indicating a good internal consistency. In the present study, a one-factor solution with factor loadings from 0.59 to 0.77 was achieved for the items. Only one study by Balc in Turkey analyzed factor loadings using a two-factor solution that led to 0.07 - 0.74 for the first factor and 0.09 - 0.83 for the second factor (20).
In the majority of the studies, the feelings and educational activates were the most and the least items influenced by AD, respectively (13). In the present study, the most and the least items affected by AD were itching and sports activities, respectively.
Our research demonstrated no relationship between the QoL and demographic features of the patients that was compatible with the Ganemo study in Sweden (22). Some surveys showed two peaks of QoL at six and 15 years old and described lower QoL in male children (21, 23). The current study revealed that patients with more severe disease had lower QoL (Pearson correlation = 0.70) that was compatible with other studies with Pearson correlations between 0.53 and 0.78 (13, 23).
The mean score of QoL in the present study was 9.44. In one study in Malaysia by Aziah et al., this number was reported to be 10 that indicated a lower QoL in comparison with our study. This difference might be due to the higher severity of the disease in their studies. The mean number of SCORAD in the Aziah study was 36 versus 31.97 in our study. In addition, the maximum severity of the disease in the Aziah study was 83 versus 57.4 in our study (24).
In the present study, AD had a significant negative effect on the QoL of 65% of the participants (38% with moderate effect, 27% with large effect) that was nearly in accordance with the Amaral study in Brazil, with a significant effect of 72% (38% moderate effect and 34% large effect) (25). A limitation of this study was the collection of data solely from one pediatric dermatology clinic as a referral center in the southeast of Iran. Therefore, it may include more severe cases that result in the lower QoL of AD children. Thus, further studies are recommended to evaluate the efficacy of treatment modalities on the QoL of patients with a larger number of patients.
5.1. Conclusion
The results of our study proved good reliability and validity of the Persian version of the CDLQI questionnaire with Cronbach’s alpha of 0.87. Therefore, it can be used in clinical trials to evaluate the efficacy of treatment in the QoL of children. Moreover, there was a positive and strong correlation between the QoL scores and the severity of the disease, but there was no relationship between demographic features and QoL of patients with AD.