1. Context
The formation of keloids, which is essentially a scar, is an abnormal wound response. Specifically, a keloid is defined as “a smooth scar with a reddish-brown fullness that occurs after inflammation/injury or without any specific causes, leading to the invasion of the surrounding tissue with a certain tendency and progressive growth. During growth and invasion, mild inflammatory erythema nodosum (which gradually leads to fullness, growth, and invasion) is clinically observed in the tissue around the tumor fullness” (1). Additionally, a keloid is a fullness formed on the skin surface due to the overproduction of scar tissue, mainly composed of collagen fibers, after prolonged wound healing for various reasons (2). Since the causes of keloids are mostly unknown, no specific therapeutic agents have been developed. In most cases, keloids are resistant to all existing treatments. This implies that to effectively treat this intractable disease, the identification of its onset mechanism and definitive factors that contribute to its formation are of great significance.
Endothelial progenitor cells (EPCs) are undifferentiated cells with high proliferation and differentiation potential that differentiate into vascular endothelial cells. In 1997, Asahara et al. were the first to report that in adult peripheral blood, CD34+ cells functioned as EPCs (3). Further, it is also well known that to repair or supplement injured vessels, EPCs differentiate into vascular cells. Fundamental studies investigating bone marrow-transplanted adult mice have also shown the presence of EPCs in the bone marrow. Pathologies such as ischemia, inflammation, wound, or tumorigenesis, cytokines (eg, granulocyte colony-stimulating factor [GCSF] and granulocyte-macrophage colony-stimulating factor [GM-CSF]), or the administration of hormones (eg, estrogen) can also cause EPCs to migrate from the bone marrow to the site of angiogenesis, which is accomplished via blood circulation where they promote angiogenesis (4, 5). In addition to CD34+ cells, AC133 and vascular endothelial growth factor receptor (VEGFR) 2 are also known as representative EPC markers.
Regarding EPCs, studies are being conducted on revascularization therapy in various areas, such as wound healing, lower-limb ischemia, ischemic heart disease, skeletal muscle repair, peripheral nerve recovery, and revascularization after spinal cord injury (6-15). The presence of abundant immature blood vessels in keloid scars suggests that EPCs possibly play an important role in keloid formation. However, there is no systematic review on the association between EPCs and keloids to date. In this review, we identify potential causes of keloid formation by examining previous studies on the association between keloids and EPCs. This review provides a novel perspective on the causes of keloids and possible treatment options. Further, we reviewed the role of CD34+ cells and related studies so as to clarify the role of EPCs in the pathophysiology of keloid formation.
2. Evidence Acquisition
We performed a thorough literature search in scholarly databases, including Web of Science, PubMed, and Google Scholar, using the following keywords: “keloid and EPC,” “keloid and CD34-positive cells,” “keloid and blood vessel,” “keloid and AC133,” and “keloid and VEGFR2.” We searched these databases for studies corresponding to the dates of individual publications up to June 2021.
3. Results
A keloid is an intractable disease; currently, apart from surgical intervention, few treatments are available to cure the disease, and its complete cure is difficult due to the lack of an effective therapeutic strategy. Treatments include tranilast (an oral medication, brand name Rizaben), steroid ointment, tape, and steroid injections. Although appropriate treatment of keloids can be expected to alleviate the symptoms, in many cases, the treatment cannot completely cure the disease, and recurrence and exacerbation are common. Since there is still a lot of uncertainty regarding the pathogenesis of keloids, very few effective treatment regimens have been developed to date. However, a better understanding of the factors underlying keloids is important to prevent the disease and develop effective drugs and treatment methods. Below, we describe the results of our search regarding the conventional causes of keloid formation, as well as the association between keloids and CD34+ cells that have been proposed to date.
3.1. Causes of Keloid Formation
Hypertrophic scars (which may include surgical scars, postoperative hematomas, wound sites without appropriate treatment, burns [types II and III], and skin graft donor sites) are independent of the patient’s physical constitution. Conversely, keloid scars (which are caused by minor injuries [eg, Bacille Calmette–Guérin vaccination, insect bite, surgical cut, and folliculitis]) are influenced by the patient’s physical constitution. Additionally, keloids are more likely to occur in non-Caucasians than in Caucasians (16). Specifically, due to genetic factors, non-Caucasians have a higher prevalence of a family history of keloid formation, and recent studies have demonstrated that single nucleotide polymorphisms (SNPs) in the Japanese population are associated with keloid formation. For example, Ogawa et al. suggested that rs8032158 may be involved in the progression of keloid and hypertrophic scars (17-19).
Normal wound healing consists of 3 phases (ie, inflammatory, proliferative, and remodeling phases). First, inflammation occurs around the wound, leading to the release of various cytokines and cell migration. Next, apoptosis of fibroblasts causes the production of extracellular matrix (ECM), primarily consisting of collagen, and the formation of granulation tissue due to neovascularization. This causes recession of the margin of granulation tissue and wound. Finally, epithelialization due to the migration of keratinocytes leads to wound healing, resulting in scar formation and wound closure (20). Even though immature scars are reddish, an increase in the number of fibroblasts over time results in apoptosis, a decrease in the number of blood capillaries, and the decomposition of the excessive ECM deposition, leading to the formation of mature, flat, and white scars.
Conversely, keloid and hypertrophic scars result in chronic and persistent dermal inflammation for reasons that are still unknown. It has been reported that the overproduction of pro-inflammatory cytokines, such as transforming growth factor β (TGF-β) and interleukin (IL)-6, leads to persistent immature reddish scars (21). Additionally, dermal inflammation leads to the overproduction of capillaries that culminate in the chronic overproduction of ECM by fibroblasts (21). While local causes of persistent dermal inflammation are mostly unknown, some of the established factors include physical stimulation (such as dynamic or mechanical stimulation), inflammation during or after removing piercings, folliculitis, and inflammation caused by folliculitis, acne, or a xenobiotic. Therefore, the treatment of keloid and hypertrophic scars may require the elimination of these physical stimulations (such as dynamic or mechanical stimulation) and inflammation after piercings (16).
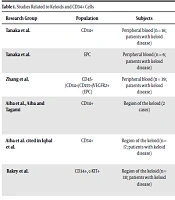
We focused on the association between stem cells and the development of keloids. In the following sections, we discuss the studies in which the role of CD34+ EPCs in the pathophysiology of keloid formation is described (Table 1).
| Research Group | Population | Subjects | Detection Method | Result | References |
|---|---|---|---|---|---|
| Tanaka et al. | CD34+ | Peripheral blood (n = 16; patients with keloid disease) | Cultures obtained; Flow cytometry | Twofold increase in patients with keloid disease (not significant) | (22) |
| Tanaka et al. | EPC | Peripheral blood (n = 6; patients with keloid disease) | Cultures obtained; Flow cytometry | Significant increase in patients with keloid disease | (22) |
| Zhang et al. | CD45−/CD34+/CD133+/VEGFR2+ (EPC) | Peripheral blood (n = 39; patients with keloid disease) | Fresh peripheral blood; Flow cytometry | Significant increase in patients with keloid disease | (23) |
| Aiba et al., Aiba and Tagami | CD34+ | Region of the keloid (2 cases) | Keloid tissue; Immunohistochemical staining | CD34+ cells absent in inflammatory keloid, CD34+ cells were expressed on the non-inflammatory keloid | (24, 25) |
| Iqbal et al. | CD34+ | Region of the keloid (n = 17; patients with keloid disease) | Keloid tissue; Immunohistochemical staining and flow cytometry | Increased CD34, CD90, and CD117-positive cell counts in extralesional skin | (26) |
| Bakry et al. | CD34+, c-KIT+ | Region of the keloid (n = 30; patients with keloid disease) | Keloid tissue; Immunohistochemical staining | CD34 and c-KIT-positive cell counts were increased by 76.7% and 100%, respectively, in keloid skin | (27) |
Studies Related to Keloids and CD34+ Cells
3.2. Keloids and CD34+ Cells
In our recent study, we evaluated the role of EPCs in peripheral blood in the pathophysiology of keloid disease. The abundance of immature blood vessels in keloid scars suggested that EPCs possibly played an important role in keloid formation (22). Further, CD34+ cells (isolated from peripheral blood samples obtained from patients with keloid disease, when compared with those from healthy subjects) showed an almost twofold increase in circulating CD34+ cell counts. However, this difference was not significant (22). Furthermore, Zhang et al. isolated EPCs from peripheral blood samples obtained from patients with keloid disease and healthy donors. The analysis of these samples via flow cytometry demonstrated a significant increase in the number of CD45-/CD34+/CD133+/VEGFR2+ cells in fresh peripheral blood samples of patients with keloids (23). Additionally, Huang and Ogawa demonstrated distortion of the systemic balance between pro- and anti-angiogenic factors in patients with keloid disease, as indicated by altered levels of circulating VEGF and EPCs (28). These studies suggest that environmental factors, which are directly or indirectly involved in the remodeling of pathological keloids, are related to the number and function of peripheral blood CD34+ EPCs.
Aiba et al. and Aiba and Tagami analyzed keloid tissue via immunohistochemical staining and observed that CD34+ cells were absent in inflammatory keloids–but present in non-inflammatory keloids (24, 25). Recently, they observed distinct subpopulations of hematopoietic and non-hematopoietic mesenchymal stem cells in keloid scars, whereby a unique population of CD34+ cells accumulated in the extra-keloid. Keloid scars provide an ecological niche for non-hematopoietic mesenchymal stem cells (26). Their data suggest that targeting and separating stem cell populations from the microenvironment of keloids may constitute a new therapy for keloid scars.
Bakry et al. focused on the role of stem cells in skin keloid development. They investigated the immunohistochemical staining of CD34 and c-KIT in 30 keloid tissue samples against normal tissue samples. Based on their observations, in 76.7% of cases, keloid sections showed increased levels of dermal stromal CD34+ cells, while 100% of cases expressed c-KIT+ cells relative to normal skin (27). Thus, several scholars have come to the conclusion that hematopoietic stem cells may be involved in keloid pathogenesis. However, given that CD34+ and c-KIT+ cells are also expressed in hair follicle stem cells and melanocytes (29-31), the relationship between keloids and epidermal stem cells (which are localized in the epidermis) cannot be ignored. Therefore, it is important to examine epidermal stem cells in keloid tissue and hematopoietic stem cells individually.
As summarized in Table 1, CD34+ EPCs were detected and found to have a higher proportion in the peripheral blood, keloid tissues, and surrounding tissues of patients with keloid disease compared with their healthy counterparts. Further, cutaneous pathological keloids may involve increased CD34+ cells in keloid tissue and systemic peripheral blood. In this review, we proposed that an increase in EPCs may be associated with keloids. Further research is needed to better understand the details of CD34+ cells/EPCs and the pathogenesis of keloids.
4. Conclusions
In recent years, the regeneration of blood vessels required for tissue and organ regeneration has received particular attention. Specifically, using EPCs containing CD34+ cells, regenerative medicines have shown remarkable wound healing, angiogenesis, and anti-inflammatory effects (6). In this review, we discussed the association between physiological CD34+ EPCs and keloids, as reported by previous studies. Based on the existing literature, it is evident that the mechanism of keloid pathogenesis and the localization and expression levels of CD34, AC133, and VEGFR2-positive cells in immature blood vessels in both the affected skin and the skin surrounding the affected area require more detailed investigations. Further, the expression of angiogenesis markers in peripheral blood should be noted as a systemic factor for the onset of keloids.
This review recapitulated previous studies related to CD34+ EPCs and their role in the intractable keloid disease; according to this review study, an excess of CD34+ EPCs may be a cause of keloid formation. We hope that this review will help facilitate the development of diagnostic biomarkers for cutaneous pathological scars and drugs/techniques/regimens to prevent, ameliorate, or cure these scars affecting the physical and psychological well-being of patients.