1. Introduction
Facial ulcers are caused by a number of disorders, including inflammatory conditions (autoimmunity, sarcoidosis, etc.), vascular diseases, and malignancies (squamous cell carcinoma and basal cell carcinoma), making it challenging in terms of diagnosis and treatment. Infectious causes include bacterial (cutaneous tuberculosis, atypical mycobacteria, leishmaniasis, and ecthyma gangrenosum), viral (herpes simplex, herpes zoster, and cytomegalovirus), and deep fungal infections (mucormycosis, chromoblastomycosis, and histoplasmosis). Mucormycosis is an uncommon fungal infection, primarily of the sinuses, which subsequently presents with cutaneous manifestations and ischemia. We here report a patient with an uncommon mucormycosis presentation, necessitating clinical acumen to be diagnosed and proactive multidisciplinary management to halt disease progression.
2. Case Presentation
A 58-year-old farmer presented with an ulcer over the left nostril from five to six days ago. He complained of nasal stuffiness and irritation, and grittiness in the left eye. On further enquiry, the patient gave a history of hospitalization due to the COVID-19 infection around one month ago. He had been treated with Inj. Remdesevir and Inj. Methylprednisolone and was on non-invasive ventilation (NIV) oxygen support for one week. After discharge, he noticed blockage in the left side of the nose and a small ulcer over the left nasal ala, which progressively enlarged in size. He was a known diabetic patient on oral hypoglycemics.
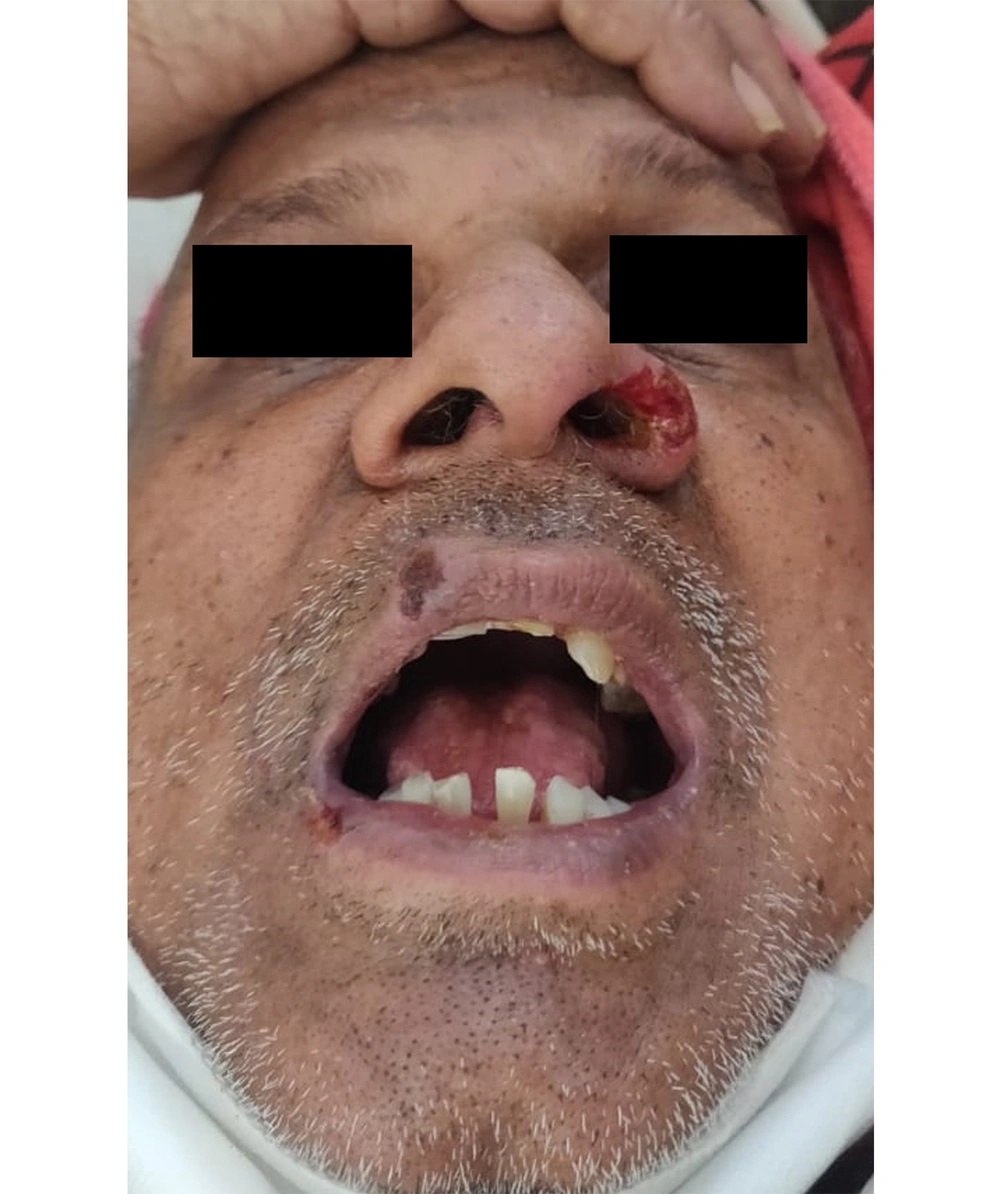
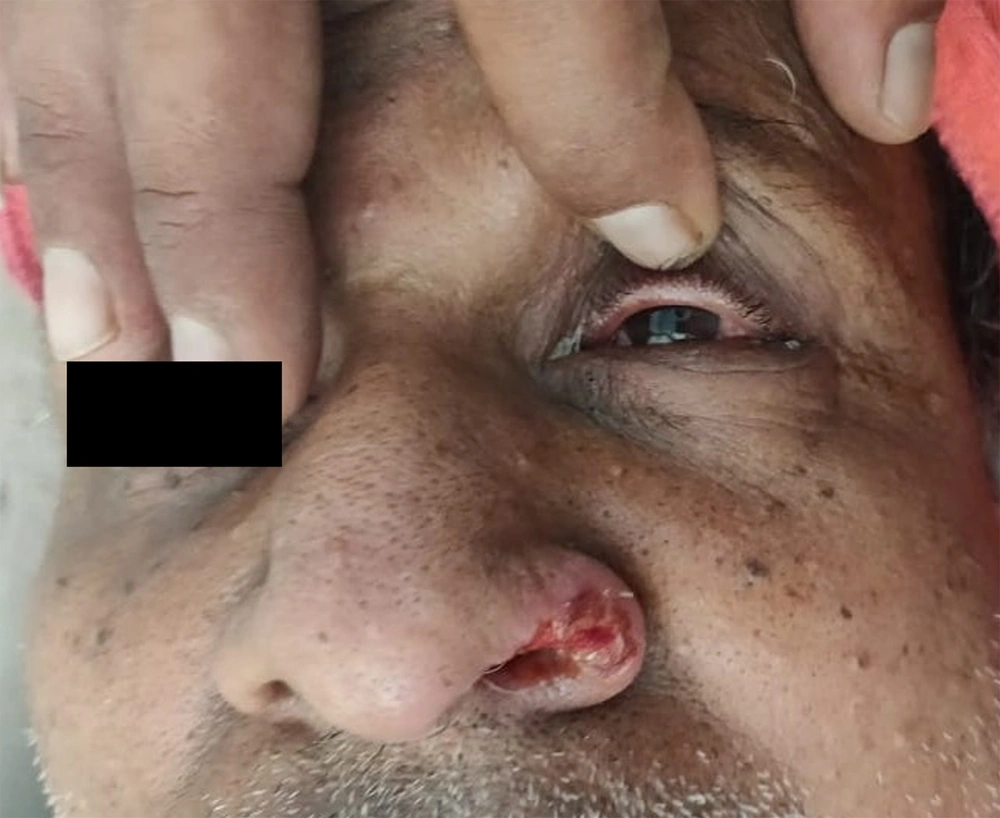
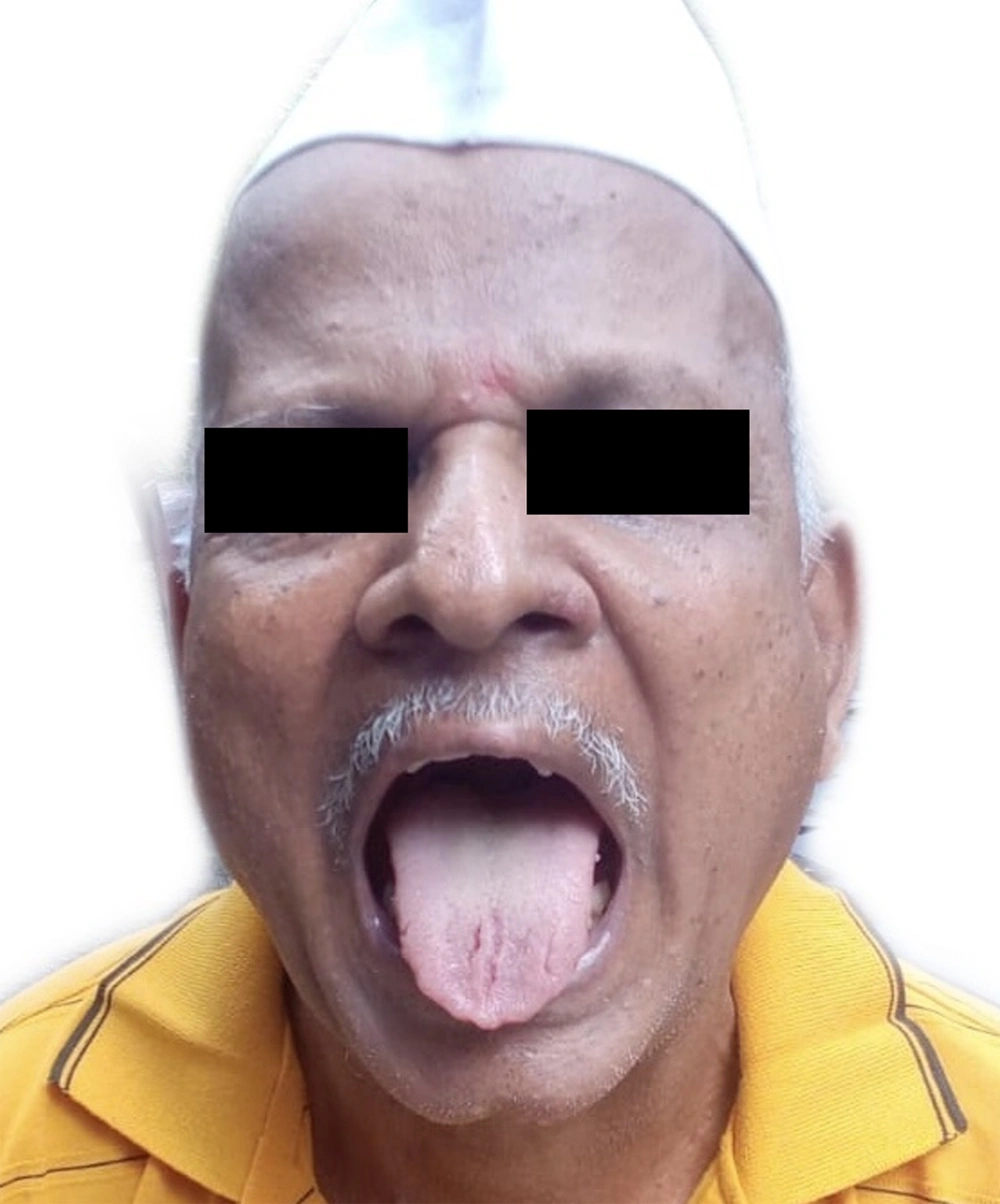
Cutaneous examination revealed a single ulcer over the left ala with the size of about 1 cm × 1 cm, irregular margins, and tissue red granulation. There were crusted erosions over the right side of the upper lip and corner of the mouth (Figure 1). The tongue was fissured, and the rest of the oral mucosa was unremarkable. The left eye showed conjunctival congestion and discharge (Figure 2). Given the ongoing pandemic and the history of a recent Covid-19 infection, a provisional diagnosis of mucormycosis was made.
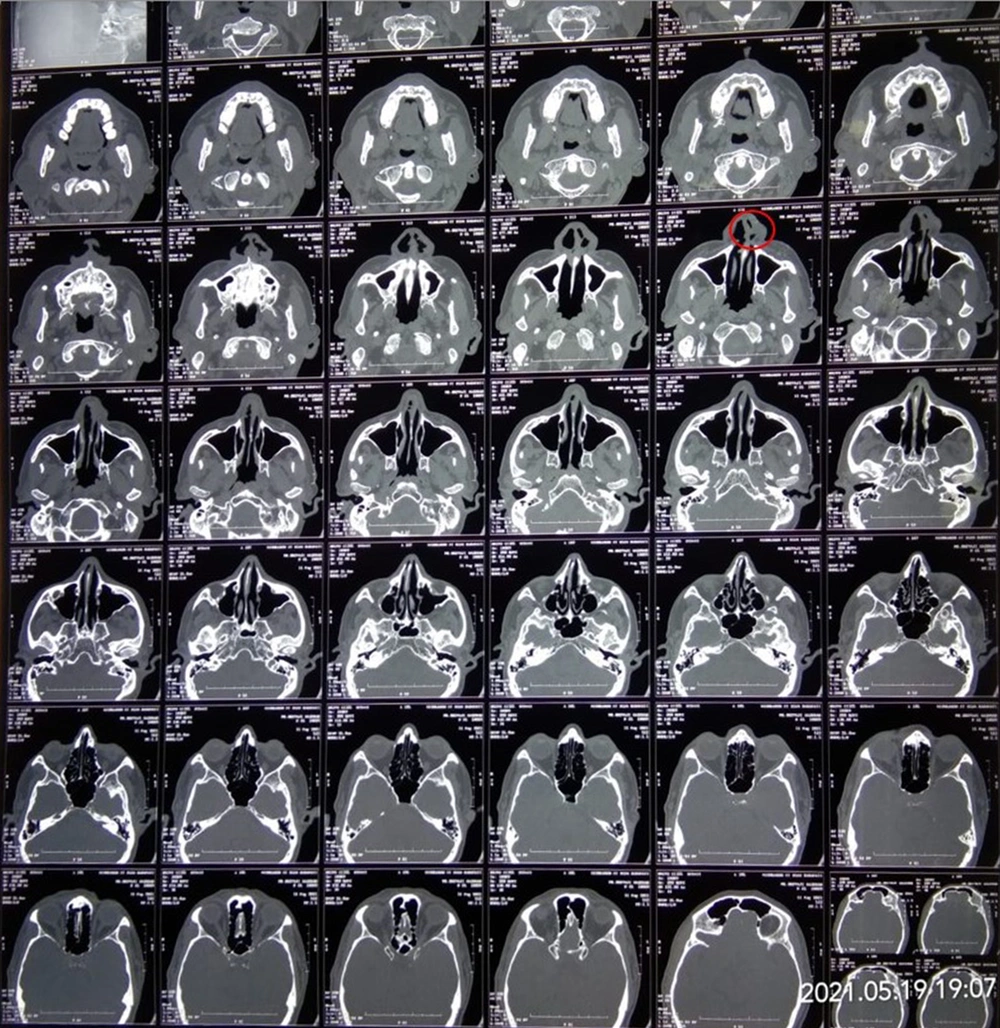
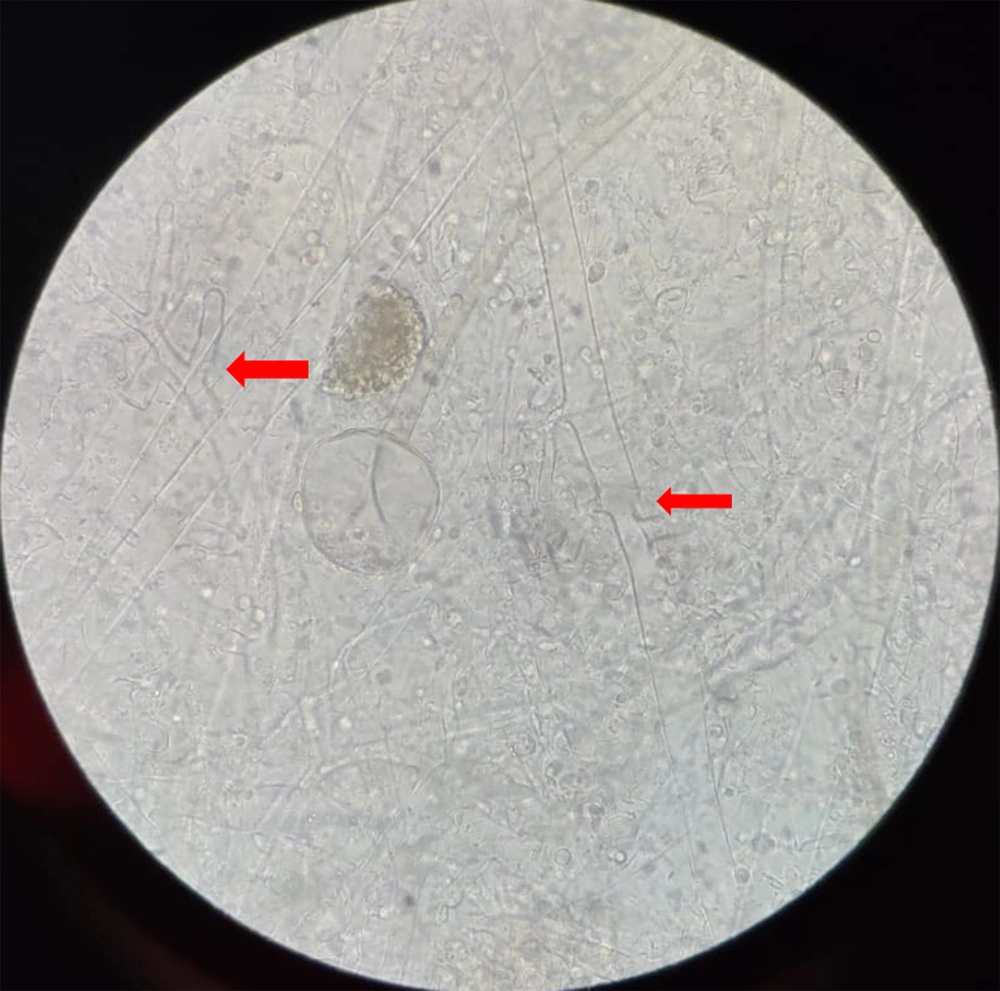
His complete hemogram and hepatic and renal function tests were within normal limits. The random blood glucose level was 300 mg/dL. Contrast Computed Tomography of paranasal sinuses (CT-PNS) showed subtle erosions over the left nasal bone, mucosal thickening of maxillary and ethmoidal sinuses, deviated nasal septum with convexity to the left, and slight hypertrophy of the middle and inferior turbinates (Figure 3). No sign of the involvement of orbit and brain parenchyma was observed on the plain and contrast magnetic resonance imaging (MRI) PNS of the orbit and brain. Potassium hydroxide (KOH) mount from nasal and labial crusts, and the ulcer showed non-septate hyaline fungal hyphae (Figure 4). Histopathological examination of ulcer margins showed nonspecific fibrocollagenous tissue partly lined by stratified squamous epithelium. Subepithelium showed mild infiltration by mononuclear cells. Periodic acid Schiff (PAS) staining for fungal hyphae and culture analysis rendered negative results. Finally, stage I rhino-orbito-cerebral mucormycosis with secondary cutaneous involvement was diagnosed.
He was treated with intravenous liposomal amphotericin-B (5 mg/kg (300 mg) in 5% dextrose over four hours for four weeks), subcutaneous Inj. plain insulin (14 units every eight hours), and Inj. insulin neutral protamine Hagedorn (NPH) (eight units every eight hours. Functional endoscopic sinus surgery (FESS) was performed. Upon monthly follow-up, there was a complete resolution of all symptoms and lesions (Figure 5).
3. Discussion
Various opportunistic bacterial and fungal infections have been associated with the COVID-19 infection caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1). Mucormycosis is an angioinvasive disease caused by the inhalation of the fungal spores of mold fungi (the genera of Rhizopus, Mucor, Rhizomucor, Cunninghamella, and Absidia, the order of Mucorales, and the class of Zygomycetes) (2). Rhizopus oryzae is responsible for 60% of human mucormycosis infections and 90% of rhino-orbital-cerebral (ROCM) cases (3). Globally, during the second wave of the pandemic, a significant number of mucormycosis cases were documented in individuals with COVID-19, and this trend was steadily increasing, especially in India. The key factor facilitating the germination of mucorales spores in COVID-19 patients is the conducive environment of prolonged hospitalization, with or without mechanical ventilation, coupled with elevated blood glucose (steroid-induced hyperglycemia, diabetes), hypoxia, acidic pH (metabolic acidosis, diabetic ketoacidosis [DKA]), iron overload (increased ferritin), decreased phagocytic activity of white blood cells attributable to the immunosuppression mediated by the SARS-CoV-2, corticosteroids, and IL-6 inhibitors, and other associated comorbidities.
The prevalence of mucormycosis is approximately 80 times higher (0.14 per 1000 population) in India compared to developed countries (4-6), and the worldwide prevalence is between 0.005 to 1.7 per million.
Primary cutaneous mucormycosis is rare. In a healthy person, traumatic injury is a known trigger. Sporadic cases have been associated with contaminated needles, bandages, and adhesive dressings. Secondary cutaneous mucormycosis is more often observed than the primary disease and usually results from rhino-orbital cerebral mucormycosis (ROCM) or disseminated infections. It has an acute onset with a fatality rate of 46% (7), and intracranial or orbital involvement and irreversible immunosuppression increase fatality to 80% (8). It usually begins with sinusitis, and its most frequent cutaneous manifestation is a necrotic eschar. Oral involvement with necrotic, black, or white ulcers may be present. Extracutaneous manifestations include fever, periorbital edema, periorbital cellulitis, proptosis, ophthalmoplegia, loss of vision, and other neurological deficits. The disease has three clinical stages. During stage I, the disease is limited to the sino-nasal area; stage II is characterized with sino-orbital involvement, and stage III is recognized with intracranial progression. Our patient had stage I disease with secondary cutaneous involvement.
Predisposing factors for mucormycosis in our patient were uncontrolled diabetes mellitus, COVID-19 pneumonitis, high-dose corticosteroid administration, NIV oxygen support, and occupational exposure to spore-contaminated soil. The early identification of the fungus is essential to establish prompt antifungal treatment. The disease can be early detected by direct KOH mount and microscopic examination, demonstrating the presence of non-septate hyaline hyphae (5 µm in width and 20 to 50 µm in length, irregular branches at right angles, and mainly at the lesional periphery). Impression smears from wound edges may also contribute to a timely diagnosis.
The diagnosis of mucormycosis is confirmed by the identification of fungal hyphae in histopathology and fungal cultures. In our case, KOH mount was positive for non-septate hyphae, which was suggestive for mucormycosis. However, negative histopathology and culture results were likely to be false due to a low fungal burden. Fungal cultures are time-consuming and low-yield, requiring a minimum duration of 4 - 6 weeks to be completed. Since histopathological examination detects the fungal invasion of tissues and vessels, as well as the host’s reaction to the fungal infection, it remains an important tool for confirmatory diagnosis. However, morphological characteristics may not be specific in most situations (9). Hence, waiting for histopathology (which may be non-specific) or culture confirmation to initiate treatment may pose a lethal threat to the patient and propensity for fulminant dissemination. So, timely diagnosis and appropriate management should not be delayed. Our case was unique and noteworthy as the pathognomonic presentation of the necrotic eschar was absent. It also underlined the utility of simple bed-side investigations like KOH mount in expediting diagnosis.
3.1. Conclusions
Clinical suspicion is essential for the prompt recognition of the cutaneous manifestations of rhino-orbito-cerebral mucormycosis during the COVID-19 pandemic. While the histopathological identification of fungal hyphae remains the mainstay for diagnosis, it should not be regarded as a prerequisite for instituting appropriate treatments.