1. Background
Psoriasis is a chronic immune-mediated skin disease in which patients with moderate-to-severe disease require lifelong control. As in other chronic disorders, adherence to a prescribed drug is a relevant topic to study. It can be defined as the degree of concordance between the patient’s behavior and the health professional recommendation. It may be influenced by different factors. Adherence can be higher when income increases, in richer geographic areas, or in psoriasis patients visiting rheumatologists (1, 2) whereas it could decrease when additional comorbidities or stressful situations take place and when self-administered medication is prescribed (3, 4). From March 2020 to date, we have faced the large-scale spread of SARS-CoV-2 virus, which has posed new challenges in the organization and operation of hospitals and pharmacy departments. Furthermore, it has questioned the management of our chronic patients in order to minimize overcrowding within the hospital, especially in those treated with immunosuppressants or immunomodulation therapy as in the moderate-severe psoriatic patient (5, 6).
At our center, we were able to run a combination of a home drug delivery system and outpatient clinic administration to avoid chronic interruption of therapy in psoriasis patients. However, we missed a centralized system to efficiently deliver drugs to patients, which could avoid additional work overload for healthcare professionals, as previously reported (7). A recent study in which 237 psoriatic patients were interviewed via phone calls about their adherence in Greece from March 15 to April 30, 2020, showed that 76.46% of patients continued their prescribed drugs (4). We believe studying the adherence in our population can be interesting as the pandemic has hit Spain harder.
2. Objectives
We calculated the medication possession ratio (MPR) with biological therapies throughout the Spanish lockdown in our hospital, and we compared the results to the same period of time in 2019 to determine the influence of the COVID-19 pandemic on their adherence.
3. Methods
This is a retrospective observational database study performed by both the dermatology and pharmacy departments of “Hospital Universitario de La Princesa”. The study included all subjects previously diagnosed with psoriasis accompanied by a biological hospital-based prescription between March 13, 2020, and May 31, 2020. For comparison, the same cohort of patients between March 13 and May 31, 2019, was included as a paired control group. All patients started the medication following the dosing regimen recommended in the pharmaceutical company label. Subsequently, in excellent responder patients, the dose was changed according to optimization guidelines.
During the study period, there was not an active call to all patients or a specific recommendation on how to act; recommendations were individualized according to the characteristics of each case. Only those who had an appointment during that period were called, and patients were examined if requested. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki.
We used our database of psoriasis patients, which includes efficacy parameters and adverse events, data on the prescribed drug posology, and a simplified medication adherence questionnaire (SMAQ). Additionally, we used the pharmacy department database, which includes for each patient in an electronic chart date of the prescribed drug collection and the number of units collected.
As a first approach, we calculated the number of units or syringes the patient was supposed to use during the period from March 13 to May 31. These data were compared with the electronic pharmacy ones to calculate the percentage of units used.
We defined adherence as the proportion of days in which a patient had biological treatments compared with the overall follow-up. In our study, adherence was determined using the medication possession ratio (MPR), defined as the number of days of supply for the drug of interest/follow-up period. Patients were considered adherent if they had an MPR of 0.8 or higher, a commonly used measure in adherence studies (2, 8-10).
3.1. Statistical Analysis
To determine MPR differences between the selected two-time intervals, a paired student t-test was performed. Comparison of MPR during the 2020 period between different treatments and drug families was carried out with ANOVA test. Results were considered statistically significant when P < 0.05. All statistical tests were performed using IBM SPSS Statistics software version 19.0.
4. Results
Our dynamic cohort consisted of 244 patients in 2020 and 228 in 2019, which were treated with either etanercept, etanercept biosimilar, adalimumab, adalimumab biosimilar, certolizumab pegol, secukinumab, ixekizumab, brodalumab, ustekinumab, guselkumab, tildrakizumab, or risankizumab.
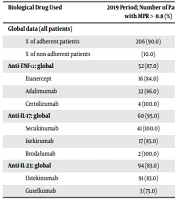
The percentage of patients adhering to different treatments individually and grouped in the two studied periods of time is shown in Table 1. Patients were less adherent in the 2020 period compared to the 2019 period [125 (51%) patients were adherent vs. 207 (91%)], showing a decrease in the percentage of adherent patients of 40.0% in COVID-19 period. Mean decrease in the percentage of used syringes was 25.3%; however, the range was wide from 11.2% for secukinumab to 43.0% for adalimumab (Table 1).
| Biological Drug Used | 2019 Period; Number of Patients with MPR > 0.8 (%) | 2020 Period; Number of Patients with MPR > 0.8 (%) | Difference in % of Used Syringes 2020 Compared to 2019 (%) |
|---|---|---|---|
| Global data (all patients) | |||
| % of adherent patients | 206 (90.0) | (51.0) | |
| % of non-adherent patients | (10.0) | (49.0) | |
| Anti-TNF-ɑ: global | 52 (87.0) | 27 (55.0) | |
| Etanercept | 16 (84.0) | 9 (82.0) | -38,4 |
| Adalimumab | 32 (86.0) | 17 (50.0) | -43,0 |
| Certolizumab | 4 (100.0) | 1 (17.0) | -24,5 |
| Anti-IL-17: global | 60 (95.0) | 33 (47.0) | |
| Secukinumab | 41 (100.0) | 20 (51.0) | -11,2 |
| Ixekizumab | 17 (85.0) | 9 (39.0) | -40,0 |
| Brodalumab | 2 (100.0) | 4 (44.0) | -18,2 |
| Anti-IL-23: global | 94 (83.0) | 65 (54.0) | |
| Ustekinumab | 91 (83.0) | 54 (56.0) | -13,5 |
| Guselkumab | 3 (75.0) | 11 (48.0) | -13,2 |
Abbreviations: IL, interleukin; MPR, medication possession ratio; TNF-ɑ: tumor necrosis factor-alpha.
a Adherent patient: A patient with an MPR > 0.8 within the studied period of time.
b Values are expressed as No. (%).
Overall, MPR that included all the patients treated with subcutaneous biological therapy was also lower in the 2020 period than in 2019 [0.70 (SD 0.38) vs. 0.95 (SD 0.25), P < 0.001]. Moreover, MPRs by treatment and drug family are shown in Table 2. In all treatments, with the exception of etanercept, certolizumab pegol, and guselkumab, differences were statistically significant between MPR in the 2019 and the 2020 periods.
| Biological Drug Used | MPR 2019 | MPR 2020 | P |
|---|---|---|---|
| Global data | 0.93 (0.20) | 0.71 (0.36) | 0.000 |
| Anti-TNF-ɑ: Global | 0.94 (0.24) | 0.71 (0.35) | 0.000 |
| Etanercept | 0.92 (0.15) | 0.72 (0.40) | 0.061 |
| Adalimumab | 0.92 (0.21) | 0.70 (0.34) | 0.002 |
| Certolizumab | 0.95 (0.12) | 0.76 (0.18) | 0.095 |
| Anti-IL-17: global | 0.96 (0.15) | 0.70 (0.33) | 0.000 |
| Secukinumab | 0.98 (0.1) | 0.73 (0.31) | 0.000 |
| Ixekizumab | 0.92 (0.23) | 0.65 (0.40) | 0.019 |
| Brodalumab | 1.00 (0.00) | 0.72 (0.27) | 0.014 |
| Anti-IL-23: global | 0.95 (0.29) | 0.69 (0.41) | 0.000 |
| Ustekinumab | 0.93 (0.23) | 0.68 (0.42) | 0.000 |
| Guselkumab | 0.93 (0.13) | 0.73 (0.36) | 0.294 |
Abbreviations: IL, interleukin; MPR, medication possession ratio; TNF-ɑ, tumor necrosis factor-alpha.
a Values are expressed as median (SD).
Tildrakizumab and risankizumab are not included in the tables as they were approved in our hospital after the period of time used as control in 2019. In the 2020 period, adherences to tildrakizumab and risankizumab were 100.0 and 86.0%, respectively.
We further studied the difference in MPR between the different treatments throughout the COVID-19 lockdown, and we did not find statistically significant differences (P = 0.984). Comparing MPR in anti-TNF-α, anti-IL-17, and anti-IL-23 family drug differences were not statistically significant either (P = 0.984).
Regarding the telephone interviews with our patients in the same time period, only a few patients decided to completely suspend the treatment without prior consultation, and all agreed to restart it after a shared talk reviewing the evidence supporting the management of their disease related to COVID-19. Many patients told us that they were trying to use a minimal amount of medication just to feel more confident, as their psoriasis was under control. We completely stopped the treatment, with the patient’s agreement, in 12 patients with COVID-19 symptoms (8 cases were confirmed by PCR or serology) for a variable length of time until they were completely recovered from the infection.
5. Discussion
Assessment of adherence is a difficult task that can be addressed using several testing methods: direct methods, more reliable and based on the measurement of drug levels in blood or urine and indirect methods, based on clinical interviews or drug dispensation count methods, such as MPR (10, 11). It is advisable to simultaneously use at least two different tools to assess adherence (12).
Regarding patients with psoriasis treated with biological therapy, their adherence has been studied mainly through the count of pharmacy refills that show notable variations, mostly due to involuntary problems like being sick or busy or just forgetting to get the new refill (13). A recent study found that adherence tends to increase with age in patients with psoriasis treated with biological therapy, but can decrease with anxiety and depression, (3) psychological symptoms that have affected not only our patients but also the general population (14).
In our study, we have demonstrated a deep impact on adherence to the situation due to COVID-19 pandemic. Other factors such as age, sex, income quintile, presence of psoriatic arthritis, among others can influence adherence. However, we consider that comparing mostly identical cohorts, at two different time points, in the same season, and in consecutive years minimizes the impact of additional confounding variables on our statistical analysis. Therefore, the observed differences in adherence can be attributed to COVID-19 pandemic-related factors. In our series, the percentage of adherent patients decreased by 40% in this 11-week period. These data contrast with previously reported results by Vakirlis et al. who studied a 6-week period in Thessaloniki, Greece, showed that 76.4% of patients were adherent (4). The WHO Coronavirus disease report of August 17 shows 28,617 deaths in Spain and 228 deaths in Greece. Since most of the decrease in drug usage seems to be driven exclusively by concerns about potential coronavirus infection, data of both studies are consistent with the epidemiological situation in their respective countries.
In addition, MPR for the 2019 period vs. the 2020 period, analyzed by drug, shows a lower adherence in the period corresponding to COVID-19 pandemic, with the exception of etanercept, certolizumab, and guselkumab. In the case of the first two treatments, due to their higher frequency of administration (every seven and fourteen days, respectively), they raised greater awareness in patients of the need for the treatment. This is not the case for adalimumab where many patients are treated with optimized doses in our cohort. In the case of guselkumab, whose frequency of administration in the maintenance phase was eight weeks, the difference between the two study periods could be explained because our cohort included six patients with optimized treatment and four of them had MPR much lower than 0.8 during the 2020 period. On the contrary, for brodalumab, differences were statistically significant between the two study periods, even though its frequency of administration during the maintenance period was two weeks. This result could be biased by the low number of patients in this group.
Finally, we fully agree with the idea of taking a non-judgmental approach to adherence (4) and we have maintained open access to patients to explain the facts about their treatment; however, nowadays evidence showed us not to discontinue immunomodulate therapy (15-17).
This shared information helped prevent uncontrolled discontinuation of medications that can cause psoriasis flare-ups, thus reducing unnecessary patient visits to the hospital. Fortunately, our patients well understood this approach of maintaining treatment by minimizing doses and adjusting our medical approach to minimize patient exposure to risky settings such as hospitals, probably because we used dose optimization of biological therapies in the past (16-18).
Despite the observation of such a decrease in general adherence between the period studied during COVID-19 blockade and the same period of the previous year (90.8 vs. 51.2%), we believe that frequent contact by phone with our patients was important to keep them informed and confident, preventing stress-induced psoriasis (17, 18).
As biological therapy portends higher medication costs to control these patients, many systematic reviews have compared the cost-effectiveness of psoriasis treatment in general, as well as that of biological therapies (19). Studying the change in cost-effectiveness was beyond the scope of our study. However, only three patients were attended in our department due to an intense psoriasis flare during the lockdown period; therefore, this peculiar situation where patients were looking forward to using the minimal dose to control their disease has probably contributed to a higher drug efficiency.
The main limitation of our study is the difficulty of measuring adherence. We have employed as the main parameter MPR, an indirect method, but easier to apply in clinical practice and widely used for the study of biological therapy adherence.
Additionally, in our study, we were not able to compare adherence between patients undergoing biological treatment and patients on conventional drug treatment. The main reason was that we could not obtain the RPM of conventional drug treatments such as cyclosporin, methotrexate, dimethyl fumarate, or acitretin because, in our country, these products are dispensed in non-hospital pharmacies.
5.1. Conclusions
In this work, we found that COVID-19 results in a much lower adherence rate of moderate to severe patients treated with biological therapy even though we were in close contact with them through mail and phone. Therefore, compliance could be worse in centers lacking dermatologists. Adherence decrease seems to affect all the different biological therapies and drug families; thus, some drugs with a higher frequency of administration are less affected, probably due to greater awareness in patients of the need for treatment. Increased experience facing SARS-CoV-2 infection will help us to further mitigate its effect on adherence in the future.