1. Context
Stem cells have the capability of self-renewing and differentiate into multiple cell lineages, such as adipocyte, chondrocyte, and osteocyte (1). They are used to repair damaged tissue because of their ability to regenerate and differentiate. In the body, stem cells recruit the resident cells to come along the damaged tissue and repair it. Thus, basically, there is a synergic effect between stem cells and cells around damaged tissue (2).
Honey has a beneficial effect on health and has often been used in medicine (3). Honey is an antibacterial, anti-inflammatory, wound healing, and regeneration of mature tissue (4). Honey's moisture content is one of its most important properties that affect the physical properties of honey, such as viscosity and crystallinity, and other parameters, such as color, taste, specific gravity, solubility, and shelf life. Honey is also composed of other substances such as proteins and organic acids (3).
Genetic engineering has been realized to engineer tissue using different materials, such as honey (5). Using single or mixed growth factors can promote high rates of tissue regeneration and repair. However, studying honey's active components is needed to determine effective single-molecule and synergistic interactions between active ingredients mediating biological effects (6).
The benefits of honey bees in the medical field include in vitro and in vivo studies. Previous in vivo studies show honey can be safely used as an effective natural remedy for topical wound healing due to its effect on enhanced tissue regeneration and recovery (7). In vitro study also shows honey bee has antibacterial effect by hurdle the H2O2 activity, viscosity, osmolality, acidity, and bioactive peptides (8). However, using honey bees for stem cell proliferation and regeneration still lacks study.
2. Objectives
In this review, we aimed to determine the effectiveness of honey bees and their ingredients on a few types of stem cells in terms of in vitro and in vivo treatment.
3. Methods
3.1. Search Strategy
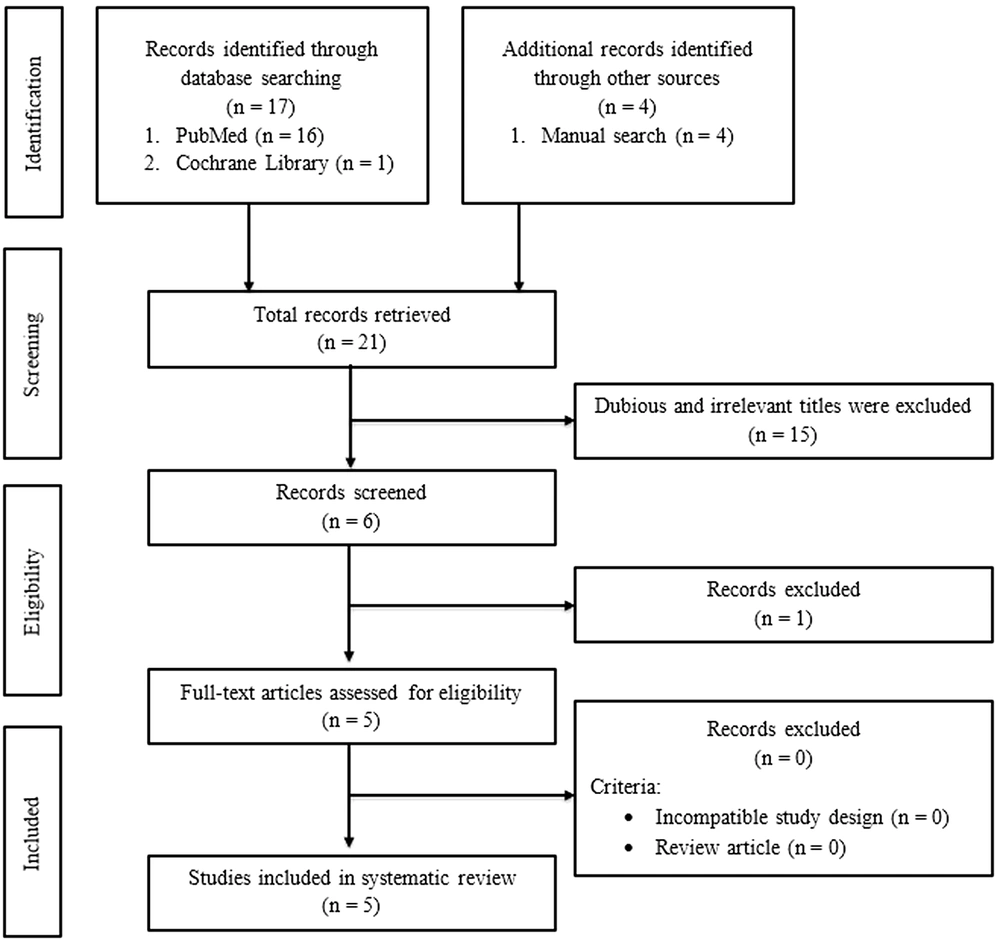
This study used the preferred reporting items for systematic reviews and meta-analyses (PRISMA) to create this systematic review. Three investigators (IR, TFAZ, M) independently searched for studies through PubMed and the Cochrane Library. This search was performed on 2021 July 30th - 31st, with search terms as follows: ("honey bee stem cells" [All Fields] OR "honey bee adipose-derived stem cells" [All Fields] OR "honey bee bone marrow mesenchymal stem cells" [All Fields] OR "honey bee umbilical cord mesenchymal stem cell" [All Fields]) OR ("honey bee" [All Fields]). Besides doing the database searching, researchers also do the manual search to enrich the result but still use the terms above. The studies used in this review were limited to English; thus, literature in other languages was excluded. Figure 1 shows the details of the literature search strategy.
3.2. Inclusion and Exclusion Criteria
Inclusion criteria are all studies that treat stem cells using honey bees in both in vitro and in vivo treatment. Exclusion criteria are studies that did not relate to the honey bee effect on stem cell growth.
3.3. Study Selection
Figure 1 shows the selection process for the included studies in this systematic review. Twenty-one pieces of literature were obtained in the first search, and 16 have irrelevant titles and/or abstracts. Thus, five pieces of literature were included in this systematic review, consisting of three in vitro studies and two in vivo studies.
3.4. Data Collection
Data collection is as follows: the aims of the study, the outcomes, sources of honey, sources of cells, types of cells, stem cells characterization, medium, culture condition, honey concentration, intervention, duration, cell number, passage, growth factor, condition/ disease, subject’s weight, subject’s age, treatments, and results.
3.5. Data Synthesis
First, data were grouped based on the study design. Further, to know the method condition and treatment, each data based on the study design is grouped into those characterizations and methods.
4. Results
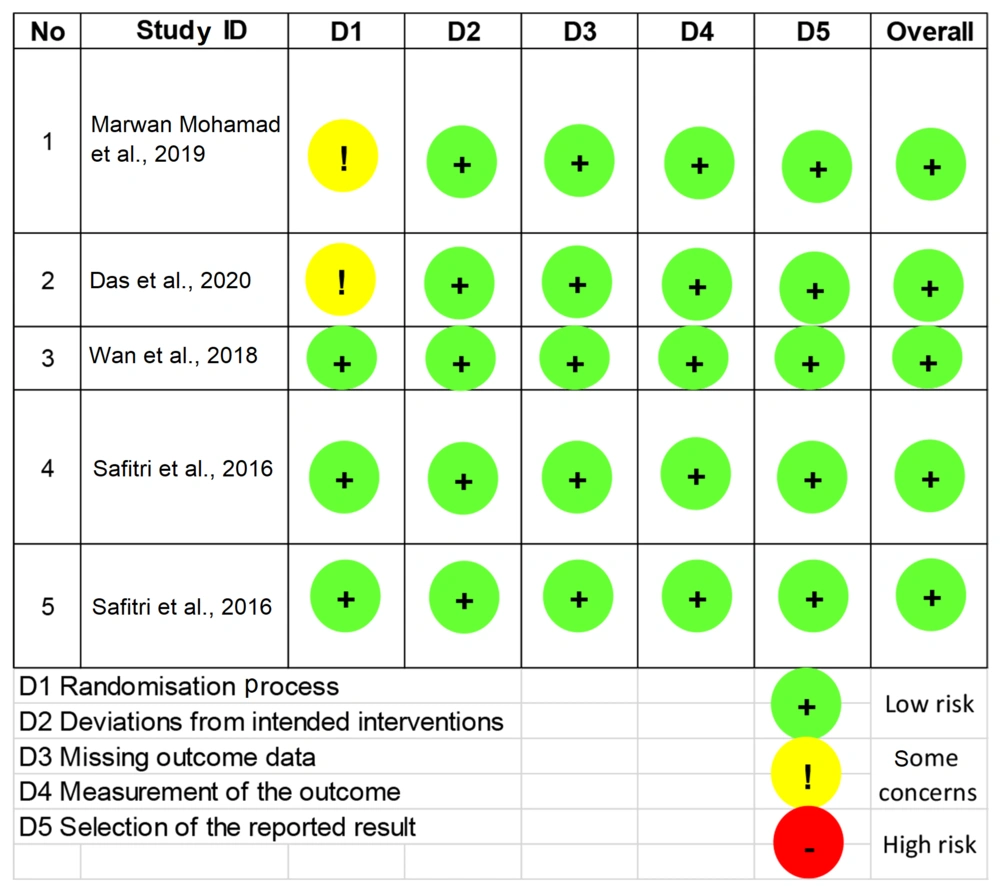
Twenty-one studies, which match the keyword, were obtained during the search. Sixteen of them were excluded due to incompatibility and incomplete data. Finally, five studies, consisting of three in vitro and two in vivo studies, were used for this systematic review. The results of the quality assessment are described in Figure 2.
4.1. In Vitro Studies Characterization
Two out of three in vitro studies used human stem cells as subjects, and the rest used mouse stem cells. The type of human stem cells used are DPSCs, with the characteristics of cluster differentiation (CD) 105 and CD90 with the fibroblast-like shape of the cells and honey from Trigona itama, and umbilical cord-derived mesenchymal stem cells (UCMSC) with the characteristics of CD105 as the positive marker and CD45 as the negative marker. The type of mouse stem cells used are embryonic stem cells (ESCs) with the characteristics of J1 and R1 and recombinant royalactin. The study aims to see if honey has a role in stem cells and shows that honey has a significant positive role in stem cells. The characteristics of in vitro studies are shown in Table 1.
| Reference | Aim | Source of Honey | Source of Cell | Type of Cell | Stem Cells Characterizations | Outcome |
|---|---|---|---|---|---|---|
| Marwan Mohamad et al., 2019 (9) | To evaluate the proliferative effect of two honeybees from Trigona spp. on DPSCs and their stability which was added in culture media. | Trigona itama | Human | Dental pulp stem cells (DPSCs) | Morphology and stem cells marker of CD105 and CD90 | This honey did not affect media stability during incubation, and it increased the proliferation rate on DPSCs in 24 hours with honeybee compared to the control. |
| Das et al., 2020 (10) | To study whether a honey-incorporated matrix can be used to reduce senescence in UCDMSCs. | Dabur honey (Dabur India Limited, India) | Human | Umbilical cord-derived mesenchymal stem cells (UCMSCs) | CD105 (positive) and CD45 (negative) | Honey uptake played an important role in reducing the ROS burden in a passage-dependent manner. |
| Wan et al., 2018 (11) | Analysis of royal actin as a potential activator of pluripotent gene networks by regulating chromatin accessibility to maintain mESC self-renewal in the absence of LIF. | Recombinant royalactin | Mouse | Embryonic stem cells (ESCs) | J1 and R1 | Royal jelly can maintain pluripotency by activating the ground-state pluripotency-like gene network. |
In Vitro Studies Characteristics
4.2. In Vitro Studies Methods
All culture conditions were set at 37°C. The treatment took 1 to 5 days in a medium with various honey concentrations and interventions. Table 2 shows the methods of in vitro studies.
| Reference | Medium | Culture Condition | Honey Concentration | Intervention | Duration | Cell Number | Passage |
|---|---|---|---|---|---|---|---|
| (9) | Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS) 10%, and penicillin/ streptomycin 5% | 37°C in 5% CO2 | 0.01%, 0.04%, 0.10%, and 0.25% | NA | 24 hours | 5 × 103, 10 × 103, and 15 × 103 | P10 |
| (10) | HiMesoXLTM mesenchymal stem cell expansion medium (HiMedia, India) | 37°C in 5% CO2 | 0.2%, 0.5%, and 1% | 12% PVA | 5 days | 1 × 104 cm-2 | P2 and P6 |
| (11) | KnockOutTM D-MEM, HyCloneTM FBS, 100 U/mL penicillin-streptomycin, 1% MEM non-essential amino acids, 1% GlutaMAXTM, 0.1 mM 2-mercaptoethanol | 37°C | 0.5 mg/mL | 0.125 mg/mL NHLRC3 | 5 days | 15000 cells/cm2 | P10 and P20 |
In Vitro Studies Methods
4.3. In Vivo Studies Characterization
Both in vivo studies used hematopoietic stem cells (HSCs) from animals as the subject. The aim was to find the effect of honey on HSCs in malnutrition male mice and female rats. Male mice stem cell characteristics are CD34 and CD45. Female rats’ growth factors are granulocyte colony-stimulating factor (G-CSF), growth differentiation factor-9 (GDF-9), and vascular endothelial growth factor (VEGF). The outcomes show honey gives a significant result between treatment groups. Table 3 shows the characteristics of in vivo studies.
| Reference | Aim | Source of Honey | Source of Cell | Type of Cell | Stem Cells Characterizations | Growth Factor | Outcome |
|---|---|---|---|---|---|---|---|
| Safitri et al., 2016 (12) | Identification of novel therapeutic methods based on autologous differentiation and mobilization Endogenous stem cells from natural honey. | NA | Male mice | Hematopoietic stem cells (HSCs) | CD34 and CD45 | NA | In this study, we found significant differences between C34 and CD45. The between-group expression also increased SSC expression and seminiferous tubule cell regeneration. |
| Safitri et al., 2016 (13) | To see the effects of honeybees on female reproduction organs. | Raw honeybee products from Batu Malang East Java, Indonesia | Female Wistar rats | Hematopoietic stem cells (HSCs) | NA | VEGF, G-CSF, and GDF-9 | This study demonstrates the expression of vascular endothelial growth factor and granulocyte colony-stimulating factor. Increased follicle Diegraaf numbers indicated increased folliculogenesis. The expression of growth differentiation factor 9 indicated the promotion of stem cell differentiation. Ovarian tissue regeneration was indicated by intact ovarian tissue with growing follicles. |
In Vivo Studies Characteristics
4.4. In vivo Studies Methods
All conditioned animals were divided into a few groups with different treatments to see the effect of honey on its HSC. Table 4 shows the methods of in vivo studies.
| Reference | Condition/Disease | Subject’s Weight (g) | Subject’s Age (Weeks) | Treatments | Results a |
|---|---|---|---|---|---|
| (12) | Malnutrition and testicular degeneration (after 5 days of food fasting, but drinking water is still administered) | 20 - 25 | 8 - 10 | 30% and 50% honey bee for 5 days | Average CD34 and CD45: T0-: 21.070 ± 1.320A; T0+: 23.010 ± 1.362A; T1: 24.360 ± 1.370A; T2: 72.180 ± 1.860B |
| Average SSCs expression (%): T0-: 86.660 ± 1.938A; T0+: 0.000 ± 0.000D; T1: 8.330 ± 0.921C; T2: 48.330. ± 0.226B | |||||
| (13) | Malnutrition and ovary degeneration (after 5 days of food fasting, they still have water to drink ad libitum using a feeding tube) | 250 - 300 | 12 - 14 | 50% honey bee | Average VEGF expression: T0-: 0.45 ± 0.85A; T0+: 0.25 ± 0.65A; T1: 2.75 ± 0.55B |
| Average G‑CSF expression: T0-: 0.75 ± 0.35C; T0+: 0 ± 0A; T1: 2.95 ± 0.43B | |||||
| Average GDF-9 expression: T0-: 2.95 ± 0.41C; T0+: 0 ± 0A; T1: 2 ± 0.45B | |||||
| Average follicle dee Graaf: T0-: 7 ± 0.85C; T0+: 0.75 ± 0.125A; T1: 5.416 ± 0.807B |
In Vivo Studies Methods
5. Discussion
Honey has the potential effect of initiating cell regeneration. Manuka honey reported that it contains bactericidal properties designed in bone engineering for cell regeneration or repair (14). Trigona spp. supplementation increased DPSC proliferation without negatively affecting their media (14). This potential effect is similar to platelet-rich plasma supplementation in adipose-derived stem cells (ADSCs) media that increased the rate of cell proliferation as well as their doubling time (15, 16). In addition, ascorbic acid that has high anti-oxidant activity showed increased proliferation (15), induced chondrogenic and osteogenic differentiation (15, 17), and delayed aging process (18).
Interestingly, honey bees also reported that it could be used as an antioxidant agent that reduces senescence in UCMSCs. Honey plays an important role in reducing ROS passage dependently (19). Recently, natural antioxidants have been extensively explored to regulate cellular behavior. Polyphenols are one of the antioxidants with the most redox properties. In this case, honey provides a proposition that reduces cell content by using various polyphenols in the engineered substrate (20). Engineered PVA-honey electro spun nanofiber matrix showed anti-inflammatory effect to regenerate the wounds (14).
Despite their potency as proliferative, antioxidant, and anti-inflammatory agents, honey bees are dependable on their concentration as supplementation. High concentrations of honey lead to toxic UCMSCs (over 3.33%) (10). Meanwhile, in vivo studies showed that honeybees increased folliculogenesis based on the expression of de Graaf in follicles. The expression of vascular endothelial growth factor and granulocyte colony-stimulating factor was increased, indicating tissue regeneration (21-23). Ovarian regeneration was evaluated by H&E staining. The honey bee group had the best outcome for ovarian tissue regeneration (24, 25). The improved appearance was similar to that of the negative control group without ovarian failure, maintaining normal growth of follicles (26).
Based on those reports, honey bees have an important role in cell regeneration. The concentration should be evaluated by using honey bees as media supplementation to increase the yield of cells or administered to the body to cure the damaged tissue.
7. Conclusions
In conclusion, honey bees increased the proliferation rate, stem cell regeneration, and wound healing. In vivo studies showed that honey bees increased tissue regeneration.