1. Context
Melasma is a common acquired pigmentary disorder, presenting as irregular light to dark brown macules on the face, mainly the cheeks, forehead, and nose (1). The term ‘melasma’ is derived from the Greek word "melas," meaning black, pointing to the color of clinical lesions. The actual prevalence of melasma is unknown, reportedly ranging from approximately 1 to 33%, depending on the location of the population (2). It is observed in people of all ethnic backgrounds and geographical areas, but populations with constitutionally darker skin, living in areas of the world with intense sun exposure, are more affected than those with lighter skin types. Among the darker skin types, it is more commonly observed in those with light brown skin types, especially Latinos and Asians (3).
Melasma is a multifactorial disorder with a variable interplay between genetic, hormonal, and environmental factors. Sun exposure, family history, and pregnancy are the most commonly observed factors associated with melasma development (2, 4, 5). On histopathology, there is an increase in epidermal melanin and melanosome number and transfer in epidermal melasma. However, melanophages are visible in the superficial and deep dermis in dermal melasma. Treatment of melasma includes topical demalanising agents with particular emphasis on photoprotection. Chemical peels and laser therapy are the other modalities used (6). Being a chronic recalcitrant dermatosis, with variable severity ranging from ‘barely visible’ to ‘severe disfigurement,' melasma has a significant effect on the quality of life of the affected individuals (7).
2. Epidemiology
Melasma has been observed to be one of the commonest dermatoses across all world countries. It was the most common pigmentary disorder and fourth among dermatoses in 546 dermatological patients in Nepal (8). Similarly, melanodermatosis, including melasma, was the third most frequent cause of skin-related consultations among 57,343 patients in a Brazilian study (9). In a study conducted in Saudi Arabia, pigmentary dermatosis represented the fourth largest dermatosis group (10). Pigmentary disorders were also the third most frequently observed dermatosis among 2,000 dermatological patients in Washington, with melasma patients comprising the second largest group (11). However, the true prevalence of melasma in the community is still underestimated due to milder disease going unreported or managed even by over-the-counter self-medication.
Globally, the prevalence of melasma is variable. As a multifactorial disorder, its prevalence has been observed from 1% in the general population to 9 - 50% in high-risk populations (12-15). This wide range of prevalence has been chiefly attributed to variations in ethnic composition and levels of photo exposure among populations residing in various geographic regions. Melasma has been reported across all ethnic groups and populations, although with variable prevalence. Pigmented phenotypes have had a higher prevalence in Southeast Asians, Middle East Asians, Mediterranean Africans, Hispanic-Americans, and Brazilians (1, 15, 16). Although it presents in 0.25 to 4% of dermatology clinic patients in Southeast Asia (17), a prevalence as high as 40% has been reported in the population (1). It is the most typical hyperpigmentary disorder among Indians (3). A prevalence of 2.9% was observed in the Arab population in Saudi Arabia compared to a 13.4 - 15.5% prevalence in Arabs residing in America (18, 19). Similarly, a prevalence of 1.5% was noted in Ethiopia (20). In another study from Brazil, 34% of women and 6% of men were noted with features of melasma (21). Among 1,000 Latino patients, a prevalence of 8.2% was noted (22, 23). Similarly, among a Latino population in Texas, the USA, 8.8% had melasma, and 4.0% gave a history of its presence (14). It was observed in 2.5% of Hispanic-American immigrants in Spain compared to only 0.5% of the Spanish population in Spain (Table 1).
| Geographical Regions | Prevalence of Melasma, % | Reference |
|---|---|---|
| Southeast Asia | 0.025 - 4 | Pasricha et al. (17) |
| Saudi Arabia | 2.9 | Parthasaradhi and Al Gufai (18) |
| Arab-Americans | 13.4 - 15.5 | El-Essawi et al. (19) |
| Ethiopia | 1.5 | Hiletework (20) |
| Latinos | 8.2; 8.8 | Sanchez (22); Werlinger et al. (14) |
| Hispanics | 2.5 | Albares Tendero et al. (23) |
3. Skin Types Involved
While melasma is observed throughout the spectrum of skin phototypes, it is more common in the middles and rare in extreme ends (24, 25). Melasma, a dermatosis due to a change in skin color, preferably affects the skin color. Therefore, it is more common in skin types III, IV, and V. Among melasma patients, as high as 90% have been observed with skin types III and IV only (26). Similar results were observed in various Brazilian studies, with most patients having skin types IV (40%) and skin type III (36%) followed by skin type V (10%) (27, 28). However, in another study, most of 188 patients had skin types IV and V (45% and 40%, respectively), with only 14% having skin type III (4).
A more common occurrence of melasma in middle skin types has been explained by the variable ability of different skin types to produce melanin on exposure to triggering factors. Due to their stable pigmentation, extreme skin types are uncommonly associated with melasma. Skin type I cannot tan and produce pigmentation on exposure to sunlight. Skin type VI cannot produce additional pigmentation since it has already produced it to its full potential (Table 2).
4. Age Distribution
Melasma has a variable age of onset. The average age range of onset varies from 20 - 30 years in some studies to 36 - 40 years in some others (27, 29). Melasma has been shown to develop earlier in the life of patients with lower phototypes. The delay in the appearance of melasma has been attributed to the photoprotective role of melanin (27, 28). Mandibular melasma specifically has been associated with later onset as compared with other types (30).
5. Sex Distribution
Melasma is more commonly observed in females than males of the same age. Generally, a female predominance of 9 - 10:1 is observed. However, the prevalence ratio of females to males is highly variable, ranging from 4:1 to 39:1 (25). A multicentric study from Brazil with 953 melasma patients had a female to male ratio of 39:1. Similarly, in a study from Singapore, a ratio of 21:1 was observed (26, 28). However, in an Indian study of 312 patients, this ratio was reported to be 4:1 (29). Several studies involving melasma patients of different skin phototypes and geographical areas have noted the involvement of women in their reproductive years, suggesting the role of hormones in the etiopathogenesis of melasma (27-33). In a study, females aged 20 to 35 years constituted more than half of the patients. In another study, 87 (87%) women were 20 - 40 years old. Similarly, in studies from India and Singapore, the mean ages of melasma development were 30 and 34 years, respectively (4, 26-29)
6. Prevalence in Pregnancy
Melasma was observed in 39.5% of female patients, among whom pregnancy was detected in 9.5% in a population-based survey in an Iranian city (34). In a cross-sectional study in Tehran, another city from Iran, a prevalence of 15.8% was observed amongst pregnant women (12). Among 224 pregnant women, melasma was identified in 10.7% in a study from Brazil (35). Similarly, in an Indian study, a prevalence of 50.8% was reported in 2,000 randomly selected pregnant women (13). A prevalence of 63.5% was noted in another Southeast Asian country (36). However, a low prevalence of 5% was observed in a group of 60 pregnant women from France (37). Increased prevalence of melasma in pregnancy and altered hormonal state with high placental, ovarian, and pituitary levels point to the crucial roles of hormones in the pathogenesis of melasma (Table 3) (38).
Women with melasma were shown to have higher estradiol hormones, Luteinizing Hormone (LH), and Follicle-stimulating Hormone (FSH), in an Indian study (39). Its prevalence also increases among women using oral contraceptive pills and hormone replacement therapy and prostatic cancer women on estrogen therapy (40). Different countries report a different prevalence of melasma during pregnancy. This could be due to genetic factors and skin type differences, further confirming the increased prevalence of melasma in more melanized skin types (37).
A significant reduction in the prevalence of melasma has been noted after 50 years of age, possibly due to the reverse alteration in the hormonal milieu compared with pregnancy. Further, aging reduces the number and activity of melanocytes (41, 42).
7. Melasma in Men
Although melasma is less frequent in men, they exhibit similar epidemiological, clinical, and histological features (31). Men constituted 25.8% of melasma patients in an Indian study, in contrast to 10% in a study from Puerto Rico, and demonstrated similar average age and disease duration to women (33.5 vs. 31.5 years and 3.5 vs. 3.1 years, respectively) (31, 43). Men have almost similar etiological factors, proving that, although female sex hormones are the predominant causal factor, they are not the only ones. Among men, like women, genetic factors, sun exposure, and outdoor work can affect the development and prevalence of the disease (43, 44). High prevalence of melasma (41%) among Indian paddy field workers further signifies the role of sun exposure in disease development (45). Sun exposure can be both a triggering and aggravating factor, with family history also having significance (4). Further, they were the most common risk factors (sun exposure 48.8%, family history 39.0%) in men, in contrast to pregnancy (45.3%) in women (43).
8. Clinical Features
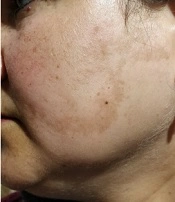
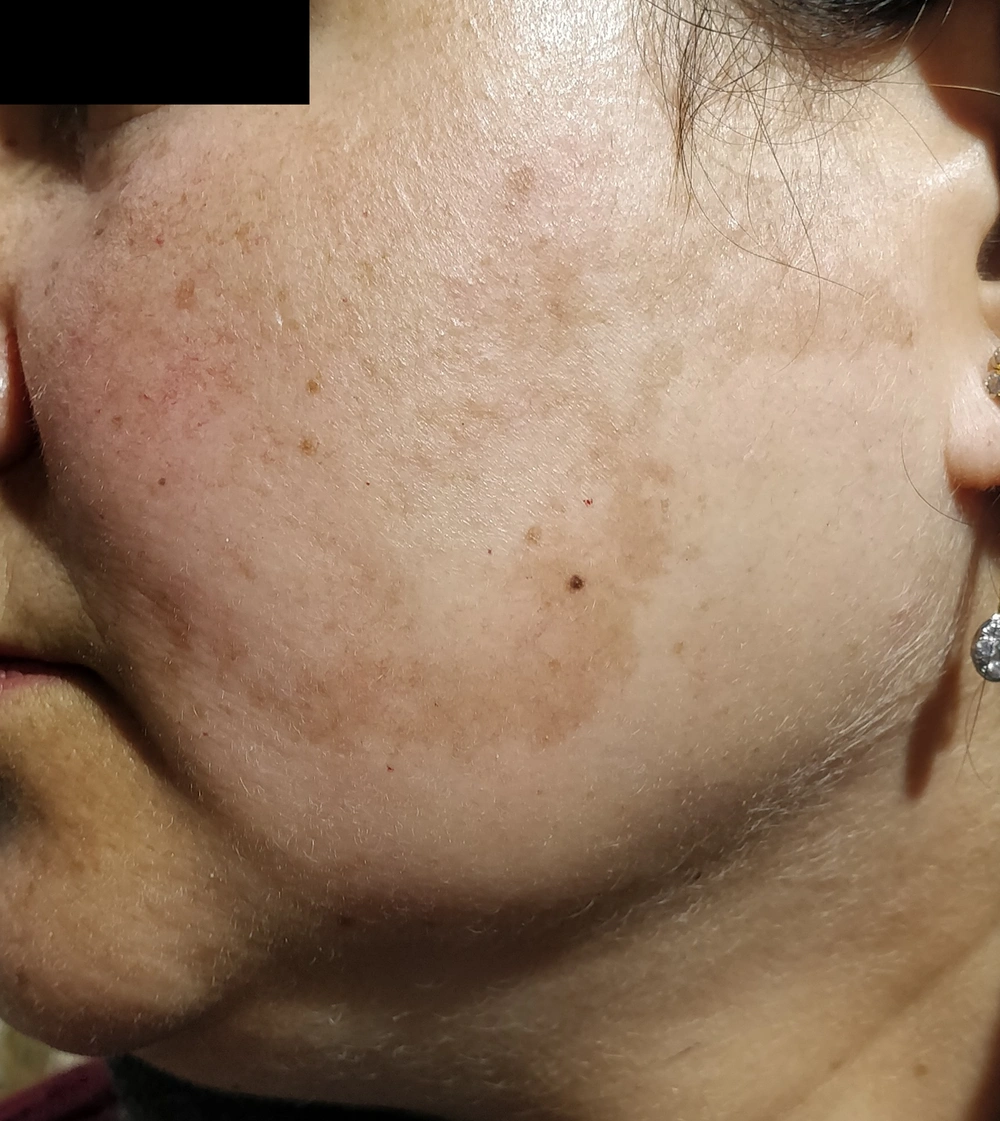
Clinical melasma patients have symmetrical ill-defined hyperpigmented macules on the photo-exposed areas, especially the face, and rarely the upper chest and extremities (27, 31). According to the distribution of these macules, melasma has been classified into three clinical patterns. The centrofacial pattern affects the central face. Forehead, nose, cheeks, upper lip, and chin are involved. The malar pattern is characterized by the involvement of the cheeks and nose. The mandibular pattern involves the mandibular dermatome predominantly.
The most common clinical pattern is the centrofacial type, followed by maxillary melasma and then mandibular melasma (Figure 1), as observed in various Indian, Brazilian, and Indonesian studies (27, 46, 47). Similar observations were reported from Tunisia, where the most common type was centrofacial melasma, accounting for 76.1% of all cases, followed by malar (22.9%) and mandibular (1%) melasma (4). However, in a study from Singapore, malar melasma was the type noted in most patients (89%). Centrofacial (8%) and mandibular (3%) melasma were seen in a minority only (26). As the malar pattern has a high co-occurrence with glabellar lesions in centrofacial melasma, it has been proposed to be considered as part of the centrofacial classification (27).
True mandibular melasma, ie, restricted to the ramus of mandible only, is rare (27, 48). In an Indian study, 1.6% of exclusive mandibular melasma cases were noted. Similarly, two (3.7%) cases were reported in a Brazilian study (27, 29). Mandibular melasma is mainly associated with older individuals and is often related to more severe exposure to sunlight. Histopathological analysis of biopsies has confirmed significant actinic damage. Therefore, it has been proposed to represent a type of poikiloderma of Civatte (4, 30). Extra-facial melasma is a new, less typical pattern. It occurs on non-facial body parts, including the neck, sternum, and forearms. Melasma affecting the upper limbs has been observed mainly among postmenopausal women, especially women on hormone replacement therapy. This type of melasma resembles facial melasma both clinically and histopathologically (49, 50).
The color of melasma is commonly tan to brown. This color is associated with excessive epidermal pigmentation. Patients with dermal melasma have blue or black macules (31). It is associated with melanophages in the superficial and deep dermis. Wood's lamp examination also helps differentiate between epidermal and dermal types. The pigmentation is accentuated in the epidermal type and not increased in the dermal type (29). Dermoscopy has also proven to be beneficial in such melasma categorization. Regular brownish appearance, irregular bluish-gray, and a combination of both are observed in epidermal, dermal, and mixed types on dermoscopy, respectively (51).
9. Melasma Assessment and Scoring
The melasma severity assessment is essential to evaluate the clinical appearance and psychosocial impact due to disfigurement caused by melasma. It is also essential to assess the therapeutic efficacy of various treatment modalities. It can be challenging, and various objective or semi-objective methods are used.
The melasma area and severity index (MASI) is a scale used to measure the melasma severity. It was introduced in 1994 by Kimbrough-Green et al (51). The MASI is the most widely used outcome measure in melasma clinical studies. This is calculated using three variables, area (A), the severity of pigmentation (P), and homogeneity (H), on the four areas of the face, forehead (f), chin (c), right and left malar cheek (rm and lm, respectively) (Table 4). The MASI allows a quantitative assessment of melasma severity. Although its inter-rater reliability, temporal stability, and consistency have been confirmed, one of the homogeneity components is problematic, and its assessment is complex. Therefore, MASI was modified after the removal of this individual component. Thus, the modified MASI (mMASI) is easy to assess and calculate, with a total score ranging from 0 to 24 (52, 53).
| Melasma Area Severity Index | |
|---|---|
| Areas and Their Scores in MASI | |
| Face is divided into four areas for evaluation of melasma severity, % | |
| Forehead (f) | 30 |
| Right malar region (rm) | 30 |
| Left malar region (lm) | 30 |
| Chin (c) | 10 |
| Area of involvement (A): Gives a numeric value from 0 to 6, as follows, % | |
| no involvement | 0 |
| ≤ 10 | 1 |
| 10 - 29 | 2 |
| 30 - 49 | 3 |
| 50 - 69 | 4 |
| 70 - 89 | 5 |
| 90 - 100 | 6 |
| Assessment of Darkness And Homogeneity | |
| Darkness (D): Gives a value of 0 to 4 as follows | |
| Normal skin color without evidence of hyperpigmentation | 0 |
| Barely visible hyperpigmentation | 1 |
| Mild hyperpigmentation | 2 |
| Moderate hyperpigmentation | 3 |
| Severe hyperpigmentation | 4 |
| Homogeneity (H) of hyperpigmentation gives a value from 0 to 4, as follows | |
| Normal skin color without evidence of hyperpigmentation | 0 |
| Specks of involvement | 1 |
| Small patchy areas of involvement < 1.5 cm diameter | 2 |
| Patches of involvement > 2 cm diameter | 3 |
| Uniform skin involvement without clear areas | 4 |
The MASI score is then calculated by the following formula:
MASI = 0.3A(f)[D(f)+H(f)] + 0.3A(rm)[D(rm)+H(rm)] + 0.3A(lm)[D(lm)+H(rm)] + 0.1A(c) [D(c)+H(c)]
The Melasma Severity Score (MSS) is another widely applied score in large trials. The MSS is divided into four grades: clear, mild, moderate, and severe. In clinical trials of therapeutic modalities, clear or mild grades are taken as ideal outcomes. It is composed of objective data and patients’ subjective assessments (54). Therefore, it has significance for both clinicians and patients, as it is easily interpreted and understood by both (55).
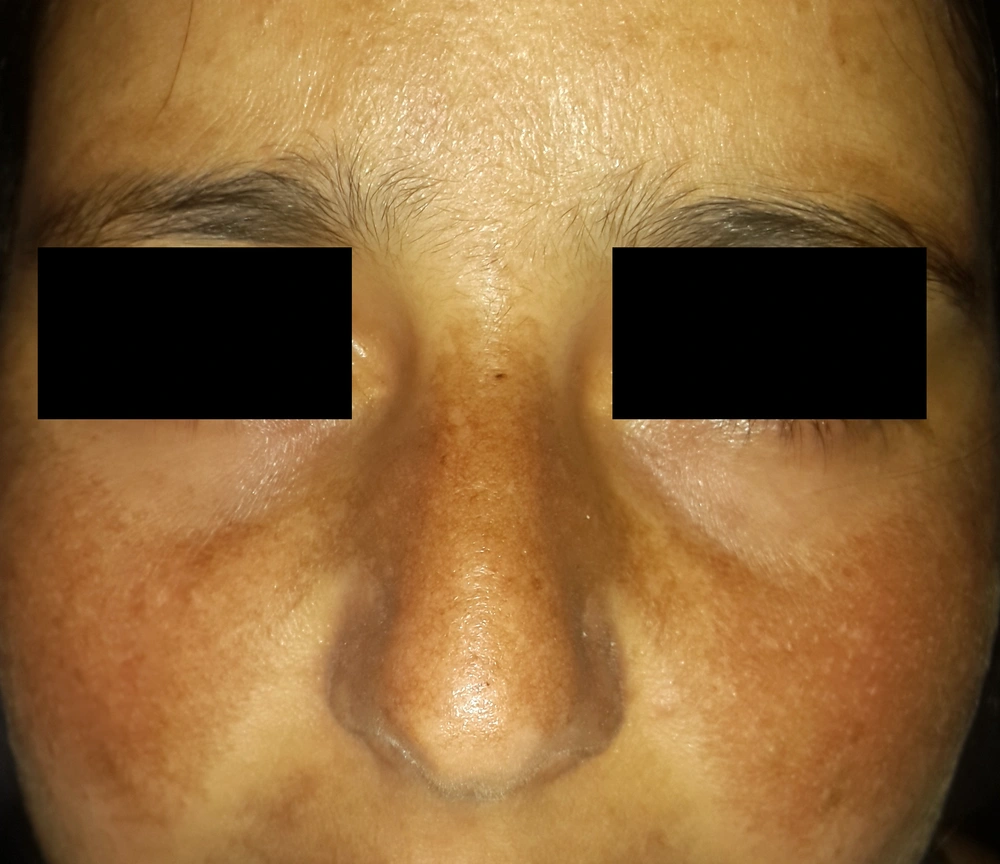
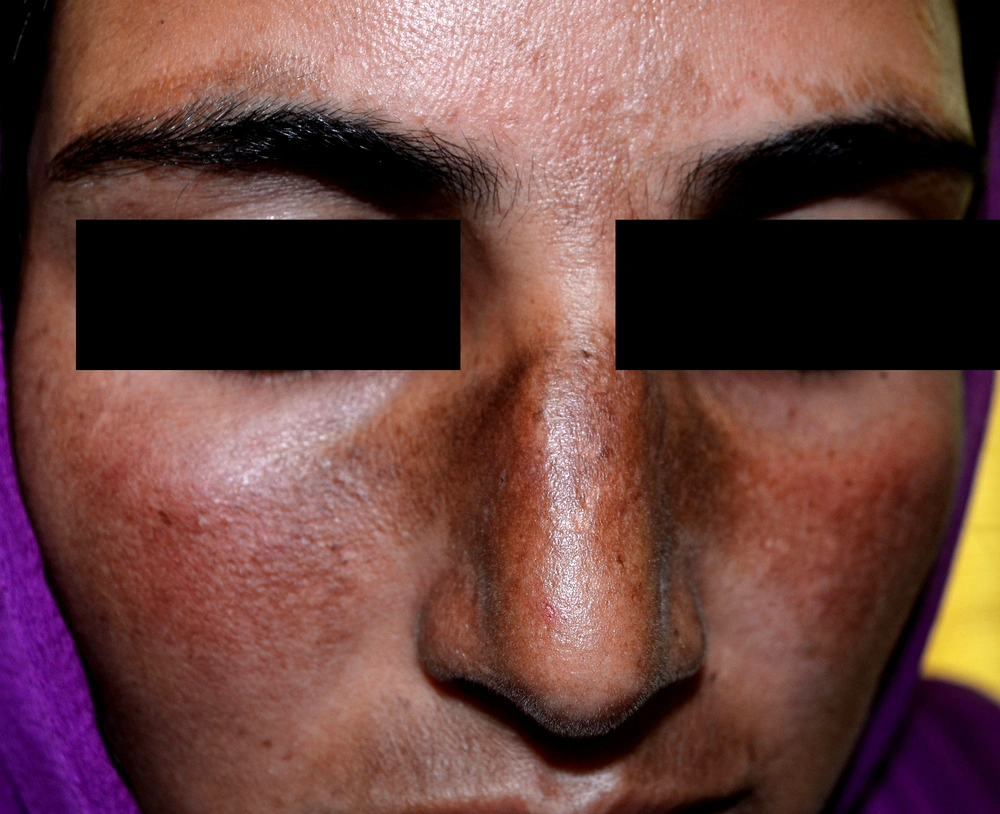
Another recently reported score for melasma is the melasma severity index (MSI), which is supposed to overcome the limitations of MASI. In the MASI score, more weightage is given to the area of involvement than the intensity of pigmentation, which ideally should be the other way round. Thus, in the MSI scoring, to give the intensity of pigmentation its due importance in assessing disease severity and psychosocial impact, the 'area of involvement score' is multiplied by the square of the 'pigmentation score.' Secondly, the nose and upper lip are assessed separately because of the non-uniformity of pigmentation in some melasma cases (Figures 2 and 3). The MSI is calculated as follows:
MSI = 0.4(a × p2)l + 0.4(a × p2)r + 0.4(a × p2)n
In this formula, “a” denotes the area of involvement, “p” the severity of pigmentation, “l” the left face, “r” the right face, and “n” the nose and upper lip. Both the variables, ie, involvement area and pigmentation severity, are scored from 0 to 4 (56) (Table 5).
| Melasma Severity Index (MSI Score) | |
|---|---|
| MSI score | MSI = 0.4(a×p2)l + (a×p2)r + 0.2(a×p2)n a |
| Scoring of pigmentation | |
| No visible pigmentation | 0 |
| Barely visible pigmentation | 1 |
| Mild pigmentation | 2 |
| Moderate pigmentation | 3 |
| Severe pigmentation | 4 |
| Scoring for the area of involvement, % | |
| ≤ 10 | 1 |
| 11 - 30 | 2 |
| 31 - 60 | 3 |
| > 60 | 4 |
a p: severity of pigmentation; a: area of involvement of face; l: left face; r: right face; n: nose; In case of uniform pigmentation over all the involved areas; MSI: max possible score = a×p2 = 4 × 42= 64
The health-related quality of life (HRQoL) tool is one of the scales used to measure the psychosocial impact of various diseases, including dermatological ones. The Melasma Quality of Life Scale (MELASQoL), a modified version of HRQoL, is used in melasma. Besides, it also helps guide treatment methods by tracking changes in patients' HRQoL. The tool includes ten questions about disease impact on the life quality rated on the Likert Scale. It emphasizes the aspects of life most adversely affected by melasma, ie, social life, recreation/leisure, and emotional wellbeing (57). The Visual Analog Scale (VAS) is another tool used for evaluating a patient’s state of mind due to a disease. A score is obtained on a “0 - 10” enumerated vertical line based on a person's emotional state. Emotions felt by melasma patients can range from “no disturbance” to “feeling of hostility.” Accordingly, the VAS value of “0” indicates no feeling of disturbance, “1 - 4” dysphoria, “5 - 6” anxiety, and “7 - 10” a feeling of hostility towards melasma (58). Other similar scales used in melasma studies are the Dermatology Life Quality Index (DLQI) and SKINDEX-16 (59-61).
10. Conclusions
Melasma is a widespread facial pigmentation disorder with a very high prevalence in people with skin color. The condition is more common in the female sex and pregnancy and is associated with a significant impact on the quality of life. The MASI and MSI scoring systems can be used to assess the melasma severity or its response to treatment.