1. Background
India accounts for approximately 60% of the global leprosy burden despite declaring elimination in 2005 (1). According to the World Health Organization (WHO) weekly epidemiological report (2020), out of 202,189 new cases reported globally, 114,451 were contributed by India with an updated prevalence rate of 0.57/10,000 (1). During January 2018-19, 1420 leprosy cases were registered at Sassoon General Hospitals, Pune, India, including 150 newly diagnosed patients. The National Leprosy Eradication Program (NLEP) has intensified efforts at eradicating this potentially crippling disease. Even after the completion of the prescribed course of multidrug therapy (MDT), many individuals have been left with characteristic deformities and disabilities of the face, hands, and feet. The propensity to leave typical deformities and the cultural beliefs associated with it have led to a profound social stigma and discrimination with psychological repercussions, even among those who have been cured.
It is estimated that there are more than three million individuals with leprosy-related disabilities in India alone (1). Merely bringing down the prevalence and incidence of leprosy may not improve the plight of those already affected. Hence, aspects like the quality of life deserve special attention. In view of the large number of afflicted persons and limited resources, it is imperative to devise strategies targeting those in greatest need. The evaluation of the quality of life before and after the initiation of any intervention can prove to be a useful outcome measure.
Previous studies from India, Brazil, and Nepal (2-4) have reported a lower quality of life among persons with leprosy compared to healthy controls. However, there is a paucity of similar data from Western India in the "post-elimination" phase, which would help initiate modifications in health care delivery. With the advent of modern amenities, better educational and employment opportunities, and consequently, higher expectations, the perception of an individual’s quality of life is bound to change. Hence, the need for frequent assessments of the quality of life has been emphasized.
2. Objectives
This study aims at documenting the effect of leprosy on the quality of life of patients and evaluating various disease-related and socio-demographic determinants.
3. Methods
A cross-sectional observational study was conducted in Sassoon General Hospital, Pune, India, a 1300 bed government-run tertiary referral center that provides health services to the surrounding urban and rural communities and is attached to Byramjee Jeejebhoy Government Medical College. Sixty leprosy patients (newly diagnosed/ initiated on or completed prescribed course of multidrug therapy) attending dermatology outpatient and inpatient departments or availing physiotherapy were enrolled after obtaining written informed consent and ensuring confidentiality. Patients under 18 years and severely ill patients who may not be able to respond to the questionnaire were excluded. Institutional Ethics Committee approval was obtained.
Medical records of these patients were scrutinized to assess disease (type and duration of leprosy, reactional episodes) and treatment (type of treatment- MDT2/MDT3/other drugs) related variables. Information on leprosy status, socioeconomic aspects, and the quality of life was collected using two independent standard semi-structured questionnaires, namely the WHO-QOL-Bref and Dermatology Life Quality Index (DLQI). The questionnaires were translated and administered in the vernacular (Marathi/Hindi) language. Patients unable to fill in the questionnaire were asked verbally, and their responses were noted.
The WHO-QOL-Bref contains four domain scores and has two individually scored items about an individual’s overall perception of the quality of life and health. It consists of 26 items scored from 1 to 5 on a Likert scale. Four domain scores are scaled in positive direction, with higher scores indicating a higher quality of life. For this study, we used the sum of the raw scores of each constituent item of the four domains, including physical health (7 items), psychological health (6 items), social relations (3 items), and environmental (8 items). The final scores of overall quality of life and each domain were calculated, resulting in final scores in a scale from 0 - 100. The overall score and that of each domain is considered good if it is more than 50% of the maximum attainable score both in a domain and in total.
The parameters taken in the physical domain are activities of daily living, dependence on medical substances and medical aids, energy and fatigue, mobility, pain and discomfort, sleep and rest, and work capacity. The parameters in psychological health are bodily image and appearance, negative and positive feelings, self-esteem, spirituality, religion, personal beliefs, thinking, learning, memory, and concentration. The social relationship domain includes personal relationships, social support, and sexual activity. In the environment domain, the parameters are financial resources, freedom, physical safety and security, health and social care accessibility and quality, home environment, opportunities for acquiring new information and skills, participation in and opportunities for recreation, leisure activities, physical environment, and transport.
The Dermatology Life Quality Index (DLQI) questionnaire is composed of ten questions, the scores for each question are interpreted as follows: (1) 0, not at all or not relevant; (2) 1, a little; (3) 2, a lot; (4) 3, very much. The final overall score of the questionnaire is interpreted as follows: (1) 0 - 1, no effect at all on patient’s life; (2) 2 - 5, small effect on patient’s life; (3) 6 - 10, moderate effect on patient’s life; (4) 11 - 20, very large effect on patient’s life; or (5) 21 - 30, extremely large effect on patient’s life. Higher scores indicate poorer quality of life.
3.1. Statistical Analysis
Data were analyzed in Open Epi Info Version 2 .3 (2009). t-test and ANOVA tests were used for the comparison of domain scores of the quality of life. A P-value of less than 0.05 was considered statistically significant.
4. Results
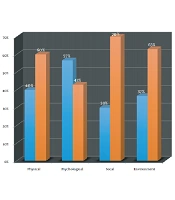
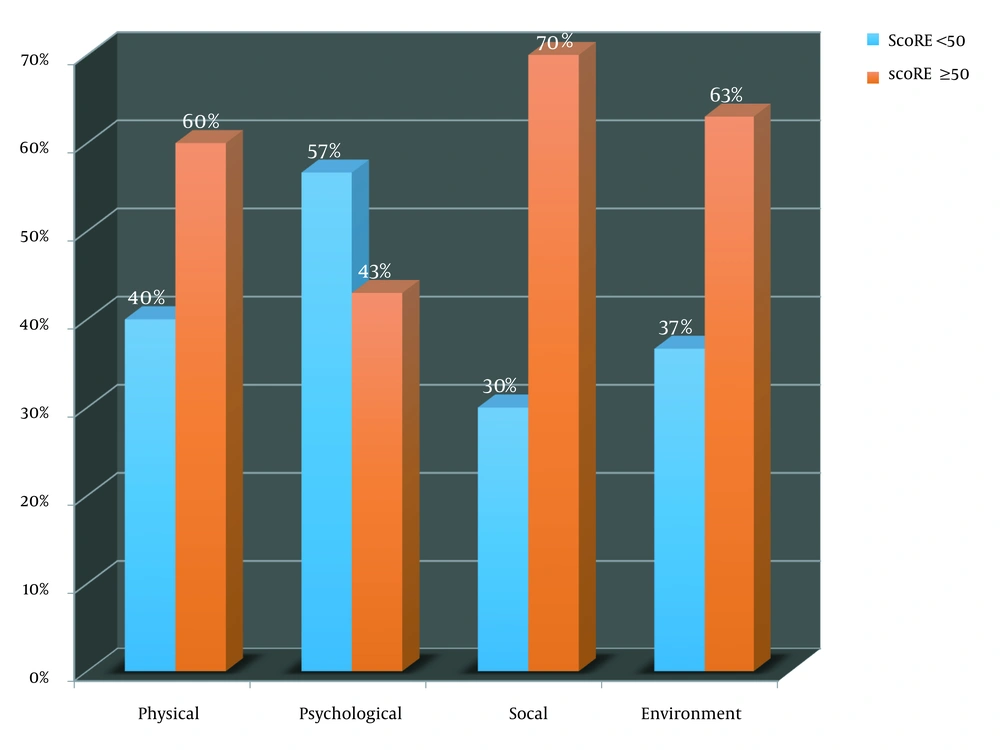
The 60 leprosy patients (Table 1) enrolled in this study comprised an equal number of males and females predominantly between 31 - 40 years of age (n = 24, 40%). Also, 60% hailed from nuclear families, while 63% belonged to Modified Kuppuswamy Score (MKS for socioeconomic status classification according to education, occupation, and income of head of family) Class IV (lower/upper lower). The borderline part of the spectrum [borderline tuberculoid (BT), mid-borderline (BB), and borderline lepromatous (BL)] accounted for 77% of the patients followed by lepromatous (20%) and pure neuritic Hansen’s (3%). Thirty-seven percent had reactional episodes comprising 30% with recurrent Type 2 reaction. Forty-seven percent were newly diagnosed, followed by relapse (27%), defaulters (13%), and released from treatment (13%). Thirty-three percent had disability (20 & 13% with WHO grade 2 and 1 respectively). The mean duration of the disease (defined as arbitrary number of years since onset of symptoms until enrolment in study) was 2.7 years (SD 0.8), and 60% had a duration of disease of less than one year. Duration between the onset of symptoms and the initiation of treatment was more than six months in 40% (mean 1.6 years, SD 0.75). Out of the 60 patients interviewed, 57% had a WHO-QOL-Bref overall score of less than or equal to 50 (Figure 1). Forty percent of the patients in the physical domain, 57% in the psychological domain, 30% in the social domain, and 37% in the environment domain had a score of less than 50, indicating a poor quality of life. Highest scores indicating a better quality of life, in all the four domains, were seen in borderline tuberculoid (BT) type, while the lowest scores in the physical, social and environmental domains, depicting a poor quality of life, were seen in lepromatous leprosy (LL). The lowest score for the psychological domain was seen with the borderline lepromatous type (BL). The lowest scores in the physical (P = 0.01) and psychological (P = 0.02) domains were seen in leprosy associated with Type 2 lepra reaction, while the highest score in these domains was seen in leprosy not associated with any reaction. Males scored higher in all the four domains indicating a relatively better quality of life. Lowest score in all the domains (social P = 0.04, environmental P = 0.03), indicating a poor quality of life was seen in patients belonging to MKS Class V (lower), while the highest score indicative of a relatively better quality of life was seen in patients belonging to MKS Class II (upper-middle).
| Variables | No. of Patients (%) |
|---|---|
| Age (y) | |
| 21 - 30 | 16 (27) |
| 31 - 40 | 24 (40) |
| 41 - 50 | 8 (13) |
| 51 - 60 | 4 (7) |
| 61 - 70 | 4(7) |
| 71 - 80 | 4 (7) |
| Gender | |
| Male | 30 (50) |
| Female | 30 (50) |
| Socio-economic status | |
| MKS class I | 0 (0) |
| MKS class II | 4 (7) |
| MKS class III | 14 (23) |
| MKS class IV | 38 (63) |
| MKS class V | 4 (7) |
| Type of family | |
| Nuclear | 36 (60) |
| Joint | 24 (40) |
| Type of leprosy | |
| BT | 20 (34) |
| BB | 12 (20) |
| BL | 20 (34) |
| LL | 6(10) |
| Pure neuritic | 2 (3) |
| Duration of symptoms (y) | |
| < 1 | 36 (60) |
| > 1 | 24 (40) |
| Time lag to treatment initiation (mo) | |
| < 6 | 36 (60) |
| > 6 | 24 (40) |
| Reactional episode | |
| No reaction | 38 (63) |
| Type 1 | 4 (7) |
| Type 2 | 18 (30) |
| WHO disability grade | |
| Grade 0 | 40 (67) |
| Grade 1 | 8 (13) |
| Grade 2 | 12 (20) |
| Current status | |
| New | 28(47) |
| Defaulter | 8 (13) |
| RFT | 8 (13) |
| Relapse | 16(27) |
Abbreviations: BT, borderline tuberculoid; BL, borderline lepromatous; BB, mid-borderline; LL, lepromatous leprosy; MKS, modified Kuppuswamy score for socioeconomic status scoring education, occupation & income of head of family); MKS class I, upper; II, upper middle; III, middle/lower middle; IV, lower/upper lower; V, lower, RFT, released from treatment.
Other statistically significant determinants of poor quality of life were: relapse (P = 0.02), Grade 2 disability (psychological domain, P = 0.04), and time lag of more than six months between symptom onset and treatment initiation (P = 0.04). The characteristics associated with poor quality of life according to WHO-QoL BREF are summarized in Table 2.
| Characteristics | Physical Domain | Psychological Domain | Social Domain | Social Domain | Overall Score |
|---|---|---|---|---|---|
| Type of leprosy | |||||
| Lepromatou leprosy (n = 12) | 41.83 ± 23.38 | 36.66 ± 26.91 | 51 ± 20.01 | 47 ± 6.29 | 44.12 ± 19.18 |
| Type of lepra reaction | |||||
| Type 2 (n = 18) | 35.55 ± 17.79 | 28.33 ± 18.13 | 49.22 ± 22.32 | 48.11 ± 6.99 | 40.30 ± 16.3 |
| Gender | |||||
| Females (n = 30) | 50.53 ± 18.64 | 40.4 ± 18.92 | 50.86 ± 19.44 | 48.93 ± 10.99 | 47.68 ± 16.9 |
| Socioeconomic status (MKS) | |||||
| Class 5 (n = 4) | 43.5 ± 10.23 | 31 ± 2.85 | 25 ± 25.38 | 41 ± 6.29 | 35.13 ± 11.2 |
| Category of patient | |||||
| Relapse (n = 16) | 45.37 ± 17.16 | 36.62 ± 16.67 | 43 ± 20.50 | 47.87 ± 11.63 | 43.22 ± 16.6 |
| WHO grade of disability | |||||
| Grade 2 (n = 12) | 44.83 ± 18.30 | 30.33 ± 18.35 | 52.16 ± 19.98 | 48.16 ± 10.80 | 43.87 ± 16.9 |
| Time lag (symptom onset to treatment initiation) | |||||
| > 6 months (n = 24) | 44.91 ± 18.31 | 33.83 ± 18.60 | 44.83 ± 19.25 | 48.16 ± 10.81 | 42.93 ± 14.8 |
a Values are expressed as mean ± SD.
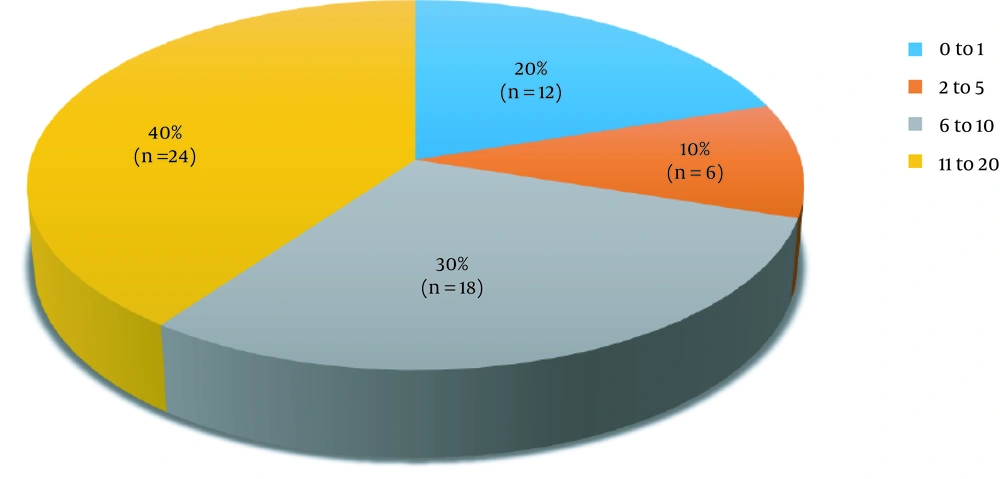
According to the DLQI (Figure 2, Table 3), the maximum (n = 24, 40%) number of patients had a score between 11 and 20, indicating a “very large effect” on the quality of life. The lowest score, indicating “small effect” was seen in borderline tuberculoid, while mid-borderline had a “moderate effect”. “Very large effect” was seen in patients of borderline lepromatous and Lepromatous type (P = 0.02). Leprosy associated with Type 2 reaction had a very “large effect” (P = 0.005), while leprosy associated with type 1 lepra reaction and leprosy not associated with lepra reaction had a moderate effect on the quality of life with the effect being more in Type 1 reaction. Female gender and relapse cases were associated with a “very large effect” (P = 0.04).
| Characteristics | DLQI | Interpretation |
|---|---|---|
| Type of leprosy | ||
| Borderline lepromatous (n = 20) | 11.7 ± 3.43 | Very large effect |
| Lepromatous (n = 12) | 10.66 ± 6.56 | Very large effect |
| Type of lepra reaction | ||
| Type 2 (n = 18) | 10.66 ± 6.56 | Very large effect |
| Gender | ||
| Females (n = 30) | 10.06 ± 5.43 | Very large effect |
| Socioeconomic status (MKS) | ||
| Class 5 (n = 4) | 11.5 ± 3.57 | Very large effect |
| Category of patient | ||
| Relapse (n = 16) | 11 ± 5.10 | Very large effect |
| WHO Grade of disability | ||
| Grade 2 (n = 12) | 11.33 ± 5.10 | Very large effect |
| Time lag (symptom onset to treatment initiation) | ||
| > 6 months (n = 24) | 11.33 ± 5.58 | Very large effect |
a Values are expressed as mean ± SD.
Other variables like age, family structure, and duration of disease were not found to have a statistically significant association with the quality of life according to either questionnaire.
5. Discussion
This study explored the effect of leprosy on the quality of life and the demographic and disease-related factors affecting it.
The majority of subjects were in the third to fifth decade of life (mean 35 years), which is the most economically productive and socially proactive age group. The mean age reported by the previous studies by Chakraborty et al. and Reis et al. was slightly higher (5, 6). The equal distribution of male and female genders in our study points to either an equal prevalence in men and women or a similarity in health-seeking behaviors. Previous studies have documented male predominance (5, 6). This variation in demographics may be due to different study settings, time periods, and socioeconomic characteristics.
The psychological domain was the lowest scoring, while the social domain was the highest, which could be explained by self-perceived stigma as against improved social acceptance. More than half of the patients had poor quality of life. The proportion of individuals with poor quality of life was higher than that documented by previous authors. A study on leprosy patients presenting to a tertiary care center in Kolkata noted that 33.33% showed poor quality of life (5). Further, low scores in physical and psychological domains with higher scores in the social domain were found (7).
According to the DLQI, leprosy had a very large effect on the quality of life. One Brazilian study showed that the majority patients had a “serious to very serious” score in the DLQI accompanied by a correlation between severity and scores (8).
In our study, the mean total score for males was higher than that of females. This is in contrast with the findings of a study conducted in the pre-elimination era wherein the mean total scores for females was higher in each domain and age group (9). This was interpreted as a greater readiness among women to accept their situation, in line with their secondary role in a male-dominated society. The findings of our study suggest either a change in the perception of quality of life in females or an actual decline. Another Indian study from Maharashtra found discrimination to be higher in female leprosy patients compared to males. They also noted a significant difference in the physical domain in males and in the psychological domain in females compared to their respective gender controls (2).
Socioeconomic status is an important potential predictor. In our study, the majority of patients belonged to Class IV (upper lower class). A statistically significant improvement in scores in social and environmental domains was noted with elevated socioeconomic status. This implies that literacy, employment, and better income have a beneficial effect on the social and environmental aspects of quality of life. A similar positive correlation was found between socio- economic class and quality of life scores by previous authors (5, 9). However, unlike the findings of the aforementioned studies, family structure did not influence quality of life in the current study.
In the present study, the majority of the subjects belonged to the borderline part of the spectrum, with more than a third presenting with reactional episodes. The lowest scores were encountered in lepromatous leprosy depicting poorer quality of life attributable to high bacillary load, systemic involvement, and great propensity for recurrent Type 2 reactions. Here, the WHO-QOL Bref and DLQI questionnaires demonstrated consensus with very large effect observed in lepromatous patients as compared to small effect in tuberculoid type. Previous studies recorded multi- bacillary leprosy in the majority of their cohort (5, 9). Proto et al. found a higher proportion of lepromatous leprosy in the Amazon region associated with a poorer quality of life. However, other demographic and disease-related variables were not elaborated (10). Similarly, higher DLQI scores for lepromatous leprosy were noted compared to controls, correlating with clinical severity but not educational level, gender, age, and disease duration (11). To corroborate this, Bottene and Reis applied the DLQI to 49 patients with paucibacillary leprosy, with the majority (63%) showing no impairment (12). Thus, earlier diagnosis and treatment initiation (before progression to multibacillary disease) alleviates the unfavorable influence on the quality of life.
Lepra reactions are acute exacerbational states occurring due to shifts in either the cell-mediated immunity (Type 1) or humoral response to circulating bacillary antigens (Type 2). In our study, patients without reactions had better quality of life scores compared to those with reactions. Out of the two types of reactions, Type 2 was associated with a worse quality of life (physical and psychological domains). This can be attributed to its plethora of recurrent manifestations, like erythema nodosum leprosum, neuritis, iridocyclitis, orchitis, and glomerulonephritis, which occur as a result of deposition of circulating immune complexes in various organs, including nerves, eyes, kidneys, and skin, associated with significant pain and functional impairment.
A study by Costa et al. involved 120 patients with reactional episodes. However, the type of reaction was not specified. Most patients reported that the disease interfered a great deal with their professional and leisure activities. The lowest rating for quality of life was observed in the physical domain and the highest in the psychological and social domains (13).
In our study, although most of the cases were new, a significant proportion presented with relapse. This alarming observation draws attention to the caveats in considering annual new case detection rate (ANCDR) as the sole indicator for evaluating the status of leprosy. Relapse was associated with a poor quality of life, while patients released from treatment reported better quality of life, probably as the latter group was free from active disease and treatment-related physical and psychological morbidity. Only 6.7% of our patients had co-morbidities like diabetes mellitus and hypertension or any other medical disorder. We were unable to find previous studies analyzing the association of reactional episodes and relapse with QoL.
The present study found that although nearly half of the patients had a time lag of more than six months between the onset of symptoms and treatment initiation, only 20% had Grade 2 disability compared to studies conducted in the pre-elimination period (9). The variation in visible deformity among different study settings could be due to earlier diagnosis and treatment initiation with better compliance and improved quality of patient care in the post-elimination phase. According to the WHO-QOL Bref, the psychological domain was affected the most in patients with Grade 2 disability as compared to those without visible deformities, which testifies to the impact of deformities and disabilities and its stigma on the psyche of the affected individual. While comparing genders, males with visible deformities had lower scores in all the domains (except physical) compared to their counterparts without visible deformities. Females with visible deformities scored less in all the domains. These findings were consistent with the results of previous studies (9). However, there seem to be conflicting reports from various parts of the world regarding the association of deformities/disabilities with quality of life. A Brazilian study using WHO-QOL Bref concluded that WHO-DG (disability grade) of leprosy did not affect the level of physical activities or quality of life, except functional capacity (14). A Bangladeshi study among 189 patients and 200 controls found that quality of life and general mental health scores of leprosy patients were worse than those of the general population (15). The factors influencing the quality of life were perceived stigma, fewer years of education, deformities, and lower annual income. Lustosa et al. demonstrated five variables co-related with health-related quality of life, namely late diagnosis, multibacillary leprosy, disability Grade 2, and prejudice (16).
The strength of our study is that it evaluated quality of life employing two different standard questionnaires, and a wide range of demographic and disease-related variables were assessed. Previous studies have used either WHO QOL-Bref (8, 11, 13, 14, 16) or DLQI (6, 10, 15) alone. The limitation is that it was not controlled.
5.1. Conclusions
This study demonstrated that leprosy continues to adversely affect the quality of life more than a decade after its official elimination in India. Determinants that contributed to the deteriorated quality of life were female gender, low socioeconomic status, delayed diagnosis and treatment initiation, multibacillary forms, Type 2 reactions, disability, and self-perceived stigma. There was good overall concordance between the WHO QOL-Bref and DLQI questionnaires. While the former is more elaborate and allows a holistic overview of quality of life, the latter is a simple practical tool for routine clinical practice. In the current setting, attention ought to be focused on early diagnosis and treatment initiation in order to prevent the development of multibacillary forms, deformities, and disabilities, along with measures to minimize reactional episodes. Upliftment of socioeconomic status by enhanced literacy and occupational rehabilitation will also contribute substantially to ameliorate quality of life.
Thus, the evaluation of quality of life deserves to become an integral part of the standard battery of tools used to assess health and well-being in leprosy and identify aspects of life (physical, psychological, or social) that could be improved by interventions.