1. Background
Seborrheic keratosis (SK) is a benign epidermal proliferation. After the fourth decade of life, SK is more common in sun-exposed areas in the Caucasians. The lesions usually develop equally in both genders. They are generally asymptomatic; however, inflamed, pruritic, or tender lesions follow trauma or infections (1-5). They usually appear as multiple brown, black, or light brown verrucous papules with about a one-centimeter diameter. The clinical subtypes of SK are dermatosis papulosa nigra, stucco keratosis, and inverted follicular keratosis. SK is mainly diagnosed clinically; however, skin biopsy is sometimes necessary to differentiate it from other skin tumors (1-5). The pathological subtypes of the disease are acanthotic, hyperkeratotic, melanoacanthoma, adenoid (reticulated), irritated, and clonal (nested) (1-5).
2. Objectives
In this study, the clinicopathological characteristics of the SK lesions in patients referred to the Afzalipour Hospital, Kerman, were evaluated.
3. Methods
This retrospective cross-sectional study examined ninety-nine skin biopsies from patients referred to the Afzalipour Hospital, Kerman, with the diagnosis of SK. The participants’ demographic features (namely age and gender), the clinical features of the lesions (site and clinical differential diagnosis), and the pathological features of the lesions were recorded. Skin biopsies were revised with two dermatopathology fellowships to confirm the diagnosis and detect the pathological subtypes. Then the correlation between clinical diagnosis and pathological diagnosis was evaluated. Furthermore, the correlation between the pathological subtypes with demographic features and the site of the lesions was evaluated.
3.1. Statistical Analysis
Data were analyzed using SPSS software version 16 (software IBM, Armonk, NY, USA). Frequency, percentage, and mean ± standard deviation were used for descriptive analysis. Independent t-test and chi-square tests were also used to assess the correlation between quantitative and qualitative variables, respectively.
4. Results
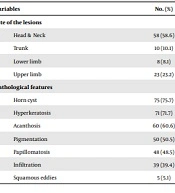
Ninety-nine skin biopsies with the diagnosis of SK were evaluated in this study. Most of the patients were female (56.6%). The mean age of the male patients was 49.79 ± 15.82 years (range 12 - 85), and the mean age of the females was 52.64 ± 16.50 years (range 16 - 80). There was no significant difference between the two genders regarding the patients’ age (P = 0.4). Most of the patients were in the sixth decade of their lives (33.3%), and sun-exposed areas were the most common site of the lesions (65.6%, Table 1).
| Variables | No. (%) |
|---|---|
| Site of the lesions | |
| Head & Neck | 58 (58.6) |
| Trunk | 10 (10.1) |
| Lower limb | 8 (8.1) |
| Upper limb | 23 (23.2) |
| Pathological features | |
| Horn cyst | 75 (75.7) |
| Hyperkeratosis | 71 (71.7) |
| Acanthosis | 60 (60.6) |
| Pigmentation | 50 (50.5) |
| Papillomatosis | 48 (48.5) |
| Infiltration | 39 (39.4) |
| Squamous eddies | 5 (5.1) |
The most common pathological subtypes were acanthotic (47.5%), hyperkeratotic (27.3%), adenoid (14.1%), clonal (7.1%), and irritated (4%), respectively. The most and the least common pathological features were horn cysts (75.8%) and squamous eddies (5.1%), respectively (Table 1). All pathological diagnoses were correlated with clinical diagnoses, and the correlation of between the first clinical diagnosis and the pathological diagnosis was 65.7%. The hyperkeratotic and acanthotic subtypes were more common in females than males (66.7% vs. 33.3% and 55.3% vs. 44.7%, respectively). Moreover, the adenoid and clonal subtypes were observed more frequently in males than females (57.2% vs. 42.9% and 57.1% vs. 42.9%, respectively); however, the difference was not statistically significant (P = 0.507, Table 2). Moreover, there was no significant correlation between pathological subtypes with either the patients’ age or sun-exposed areas (P = 0.257 & P = 0.05, respectively) (Tables 2 and 3). The most common clinical diagnoses in were SK (65.7%), wart (13.1%), melanocytic nevus (6.1%), basal cell carcinoma (BCC, 5.1%), melanoma (3%), skin tag (2%), lichenoid keratosis (2%), lentigo (1%), and Bowen’s disease (1%), respectively. The most common clinicopathological correlation was observed in sun-protected areas rather than sun-exposed ones (74% and 27%, respectively); however, the difference was not statistically significant (P = 0.119).
| Variables | Total | Hyperkeratotic | Adenoid | Acanthotic | Clonal | Irritated | P-Value |
|---|---|---|---|---|---|---|---|
| Age (y) | 0.25 | ||||||
| 10 - 20 | 7 (7.1) | 2 (7.4) | 1 (6.7) | 4 (8.5) | 0 (0) | 0 (0) | |
| 21 - 30 | 7 (7.1) | 1 (3.7) | 2 (13.3) | 4 (8.5) | 0 (0) | 0 (0) | |
| 30 - 40 | 10 (10.1) | 1 (3.7) | 2 (20) | 6 (12.8) | 1 (14.3) | 0 (0) | |
| 41 - 50 | 18 (18.2) | 6 (22.6) | 2 (13.4) | 7 (14.9) | 2 (28.6) | 1 (25) | |
| 51 - 60 | 29 (29.3) | 4 (14.8) | 2 (13.3) | 19 (40.4) | 2 (28.6) | 2 (50) | |
| 61 - 70 | 13 (13.1) | 4 (4.8) | 2 (13.3) | 4 (8.5) | 2 (28.6) | 1 (25) | |
| 71 - 80 | 14 (14.1) | 9 (33.3) | 2 (13.3) | 3 (6.4) | 0 (0) | 0 (0) | |
| 81 - 90 | 1 (1) | 0 (0) | 1 (7.1) | 0 (0) | 0 (0) | 0 (0) | |
| Sex | 0.5 | ||||||
| Male | 43 (43.4) | 9 (33.3) | 8 (57.2) | 21 (44.7) | 4 (57.1) | 1 (25) | |
| Female | 56 (56.6) | 18 (66.7) | 6 (42.9) | 26 (55.3) | 3 (42.9) | 3 (75) |
a Values are expressed as No. (%).
| Variables | No. (%) |
|---|---|
| Sun-exposed | |
| Acanthotic | 33 (50.8) |
| Hyperkeratotic | 18 (27.7) |
| Adenoid | 10 (15.4) |
| Irritated | 3 (4.6) |
| Clonal | 1 (1.5) |
| Sun-protected | |
| Acanthotic | 14 (41.2) |
| Hyperkeratotic | 9 (26.5) |
| Clonal | 6 (17.6) |
| Adenoid | 4 (11.8) |
| Irritated | 1 (2.9) |
5. Discussion
In this study, the patients were mainly in the sixth decade of their lives (33.1%), and this finding is in line with the findings of other studies (6-9). Furthermore, in the present study, 7.1% of the lesions emerged in the patients aged below 20 years. In Gill et al.’s study (10), the percentage was roughly double (15.7%). This can be explained by inconsistencies in the type of the studies, geographical areas, ethnic and genetic backgrounds, cultural differences, percentage of sun-exposure, and type of covering (10).
In this study, there was no significant difference between males and females regarding the prevalence of SK. Furthermore, in line with previous studies (10-12), the male and female patients were almost homogenous in terms of age.
Although the exact pathogenesis of the SK was not well-understood, factors such as sun exposure, genetic predisposition, and genetic mutation in FGFR3 due to ultraviolet (especially in adenoid type) were also considered. Most of the lesions were located in sun-exposed areas (65.6%) in the present study. In other studies in Iran and South Korea, most of the lesions were similarly located in sun-exposed areas (78.3% and 56.3%, respectively) (7, 9). Furthermore, there was no significant difference between pathological subtypes and sun-exposure in the present study. In contrast, Roh et al. reported the higher prevalence of the adenoid type in sun-exposed areas (7).
In this study, all pathological diagnoses were correlated with clinical diagnoses, and the correlation between the first clinical diagnosis and pathological diagnosis was 65.7%. Moreover, the smallest clinicopathological correlation was associated with lesions on the sun-exposed areas. In these areas, lesions can be misdiagnosed with non-melanocytic skin tumors [eg, BCC, squamous cell carcinoma (SCC), and melanoma]. The most common differential diagnoses in the present study were wart (13.1%), melanocytic nevus (6.1%), and skin cancers, including BCC (5.1%) and melanoma (3%). Roh et al. demonstrated a disagreement between clinical diagnosis and pathological diagnosis in 27.36% of the patients. The most prevalent clinical diagnoses were wart (42.3%), BCC (11.5%), and SCC, dysplastic nevi, or compound nevi (each 7.7%). This finding was to some extent consistent with the findings of the present study. Likewise, in Roh et al.'s study, higher disagreement between clinical and pathological diagnoses was observed in sun-exposed sites (P = 0.043) (7).
The most common pathological subtypes in the present study were acanthotic and hyperkeratotic types (47.5% & 27.3%, respectively). This finding is in line with those of the the previous studies (8, 9). In this study, the adenoid type was the third pathological subtype (14%); however, the same finding was not recorded in other studies (7, 9, 12). The clinical resemblance between the adenoid type and pigmented lesions such as solar lentigo and lentigo maligna can justify more biopsy of the lesions in the present study (8, 9).
In this study, the most common pathological features were horn cyst (75.8%), acanthosis (71.7%), and hyperkeratosis (60.6%), respectively. In India, Alapatt et al. reported pigmentation (78%), papillomatosis (68%), acanthosis (56%), and hyperkeratosis (54%) as the most common pathological features. The higher percentage of pigmentation in their study compared to the present study (50.5%) can be explained by the darker skin of Indian patients and the large number of clinical subtypes of dermatosis papulosa nigra (26%) (12).
The major limitations of this study were retrospective design and relatively low sample size. Further prospective studies with a larger sample size are recommended.
5.1. Conclusions
In this study, most of the patients were female and in the sixth decade of their lives. The most common pathological subtype was the acanthotic type, and the most prevalent pathological feature was horn cyst. There was no correlation between the pathological subtype and the patients’ demographic features.