1. Background
Platelet-rich plasma (PRP) is an autologous blood product defined by a concentration of platelets higher than baseline, rich in growth factors and cytokines involved in tissue restoration and immunomodulation. Moreover, it is a fairly straightforward and minimally invasive procedure, in addition to its low cost and safety, presenting no risk of immunological reaction (1).
Currently, enough evidence supports the application of PRP for the treatment of many orthopedic disorders. Its use is associated with restrained inflammation and pain, improved articular function, and possibly, cartilage regeneration, with reported superior results than hyaluronic acid and corticosteroids (2-7).
In the daily practice, our team treats patients suffering from different pathologies of the musculoskeletal system, including patients with hemophilia. In the latter, the use of PRP for the management of chronic synovitis has exposed fulfilling results in reducing the number of bleeding episodes, pain lessening, and decreasing joint swelling (8, 9).
Complications after intramuscular or intra-articular injection (such as abscesses, skin necrosis, or septic arthritis) are rare, but may occur by different mechanisms such as iatrogenic inoculation (10).
2. Objectives
The aim of this study was to evaluate the incidence of infection and/or complications events in patients with musculoskeletal disorders of diverse etiologies undergoing intra-articular or soft tissue PRP injections.
3. Methods
Platelet-rich plasma injections were performed at Juan A. Fernandez Hospital in Buenos Aires, Argentina. A total of 923 PRP injections were administered in 513 patients included in this study; 59% of the total population were men, and 41% were women. The mean age was 43.9 (7-91; SD = 20.8) years old. Three hundred and fifty-four (69%) patients presented common orthopedic disorders, while 159 (31%) presented hemophilic synovitis/arthropathy. In total, 83 patients presented bilateral conditions, 50 of whom were patients with hemophilia.
The study followed the principles of the Declaration of Helsinki and was approved by the Institutional Ethics Committee according to law 3301, decree 58/2011, resolution 485/MSGC/2011, Buenos Aires City Government. All patients signed informed consent.
The inclusion criteria were male and female patients between three and 95 years old with clinical and imaging diagnosis who showed no symptomatic relief after conservative treatment with analgesics and physiotherapy and gave consent to be included in the PRP treatment protocol. The exclusion criteria were as follows patients under three years of age, history of neoplasm, presence of any active infection, presence of any wound over the site to be injected.
3.1. PRP Preparation
Peripheral whole blood was collected with a BD Vacutainer® collection set, including butterfly needle, holder, and glass tubes with trisodium citrate, citric acid, and dextrose (ACD, BD vacutainer®, New Jersey, USA). In patients with hemophilia, coagulation factors were loaded after blood extraction according to the indication of the treated hematologist. The blood was centrifuged for 8 min at 450xg in an oscillating rotor centrifuge (Presvac, Buenos Aires, Argentina). The platelet-rich fraction was separated under sterile conditions in a class II biological safety cabinet (Esco, Singapore, Republic of Singapore), and placed in a sterile syringe.
3.2. PRP Administration
Platelet-rich plasma injections were performed in the procedure room. The affected area was previously sanitized with povidone-iodine solution and covered with sterile field drapes. PRP was injected freshly within the first hour after preparation, according to the musculoskeletal lesion, using a 21G needle. A mean volume of 4.3 mL (1 - 19 mL, SD: 1.8) of PRP was injected, according to the size of each type of joint. For intra-articular injection, a mean volume of 4.0 mL (1 - 10 mL, SD: 1.6) of PRP was injected. For soft tissue procedure, a mean volume of 4.8 mL (2 - 19 mi, SD: 2.0) of PRP was injected. All procedures were performed without anesthesia and under ultrasound image, no antibiotics were administered. In patients with hemophilia and synovitis or arthropathy, joint aspiration was done before PRP injection, if required. Patients were advised not to use non-steroidal anti-inflammatory drugs (NSAIDs) for 15 days before and after the procedure.
3.3. Statistical Analysis
Descriptive analysis was presented as frequency (percent) of interventions and was based on association assessment between the site of injection or the type of intervention (intra-articular or soft tissue injection) and the presence or absence of a bleeding disease. Data were analyzed with IBM SPSS Statistics Software, using Pearson’s Chi-square test and Cramer’s V test. A significance level of 0.001 was used.
4. Results
A total of 923 injections of PRP were performed in 513 patients; 431 presented one affected anatomical site, while 82 patients presented more than one affected site. Regarding the number of interventions, 305 patients (59.5%) received a single PRP injection, 123 patients (24.0%) received two injections, 34 patients (6.6%) received three injections, and 51 patients (9.9%) received four or more injections, with an interval of at least two weeks between the first and second doses.
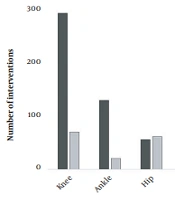
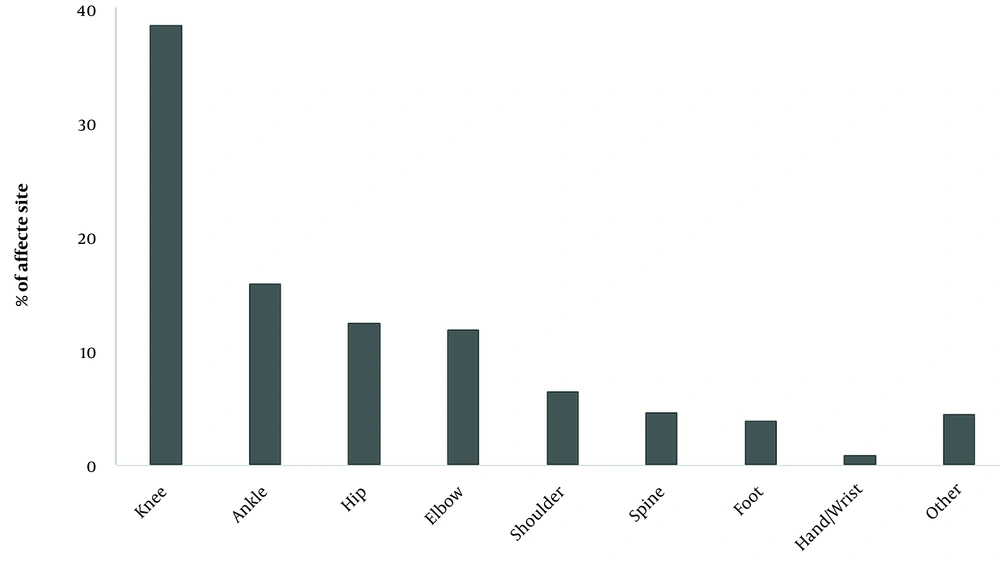
Of the total procedures performed, 583 were intra-articular injections (63.2%), and 340 were performed in soft tissues (36.8%). It should be noted that intra-articular injections were more common in patients with hemophilia, representing 70.7% of all intra-articular injections, while soft tissue injections were carried out only in patients without hemophilia (Table 1). Among the whole study population, the most commonly affected anatomical sites were: the knee (38.9%), the ankle (16.0%), the hip (12.6%), and the elbow (12.0%) (Figure 1). A higher prevalence of ankle and elbow disorders was observed in patients with hemophilia compared to patients without hemophilia. There was a statistically significant relationship between the frequency of these affected anatomical sites and the presence of a bleeding disorder (P-value < 0.001).
| All Patients | With Hemophilia | No Hemophilia | |
|---|---|---|---|
| Patients (n) | 513 (100.0) | 159 (31.0) | 354 (69.0) |
| Male | 303 (59.1) | 157 (51.8) | 146 (48.2) |
| Female | 210 (40.9) | 2 (1.0) | 208 (99.0) |
| Mean age (SD) | 43.9 (20.8) | 27.0 (11.7) | 57.5 (15.9) |
| Min-max age | 7 - 91 | 7 - 69 | 16 - 91 |
| PRP injections | 923 (100.0) | 412 (44.6) | 511 (55.4) |
| Intra-articular | 583 (63.2) | 412 (70.7) | 165 (28.3) |
| Soft tissues | 340 (36.8) | - | 340 (100.0) |
aValues are expressed as No. (%) unless otherwise indicated.
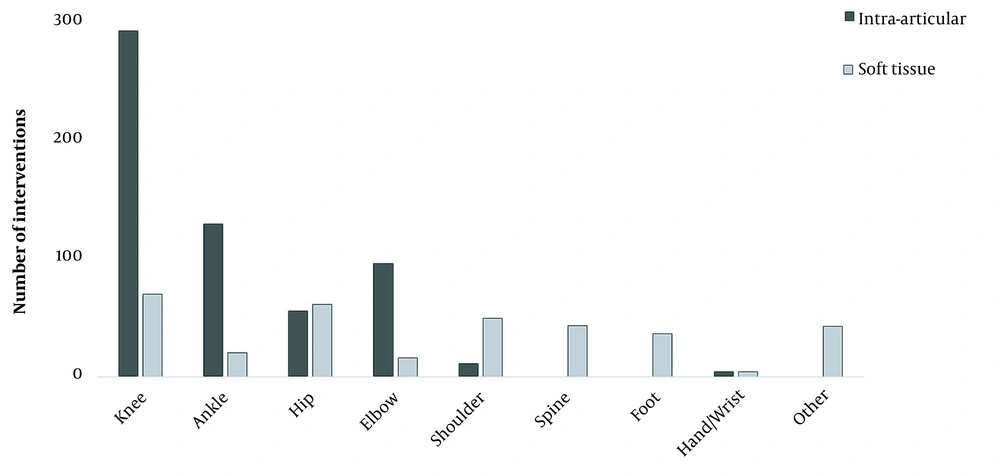
Moreover, PRP injections of the knee, ankle, and elbow were mostly intra-articular, while interventions of the hip and shoulder were mostly soft tissue injections, and all interventions assigned to the spine or foot were performed in soft tissues (Figure 2).
Intra-articular injections were more common in patients with hemophilia compared to patients without hemophilia. The relationship between the presence of hemophilia and the frequency of intra-articular interventions was also statistically significant (P-value < 0.001). Among patients with hemophilia, 13 of them presented HCV infection, one of them presented HIV infection, and three were coinfected with HCV and HIV. Additionally, 12 patients presented inhibitors.
Sometimes, pain after the injection was observed, but it responded to analgesic treatment with paracetamol. No complications in none of the 513 patients treated with PRP were observed during blood extraction or after PRP injection. No infections occurred in the site of injection, even in patients with hemophilia.
5. Discussion
There are currently different treatment options available for common orthopedic conditions, including physiotherapy, life-style modifications, and pharmaceuticals, such as NSAIDs, opioids, and glucosamine/chondroitin supplementation. Steroids and hyaluronic acid injections are commonly used as well, especially for osteoarthritis. Conservative treatment aims at the prevention or deferment of frequently performed surgical interventions such as arthroscopies, osteotomies, and total joint replacements (11). However, the use of medical therapy and anti-inflammatory drugs has a short-term effect in the alleviation of pain.
In this context, PRP emerges as a promising biologic treatment with many benefits: it is an autologous product, rich in growth factors and cytokines capable of influencing the immune response and reducing local inflammation, as well as promoting regenerating mechanisms in injured tissues. The application of PRP can help tissue recovery by stimulation of cell proliferation, angiogenesis, and collagen synthesis (12-16). Furthermore, its safety and low risk of complications after administration are important reasons for its increasing use in the recent years for the management of different pathologies.
Some authors have mentioned local minor adverse events after PRP injections -such as mild pain and effusion- that were self-limiting and remitted within a few days (11, 17, 18), except for one case that lasted up to two weeks (19). One study comprising 32 patients undergoing intra-articular PRP treatment for knee osteoarthritis, reported two mild and two moderate to severe cases of acute inflammation, in which infection was ruled out, and all patients recovered with functional outcomes after the corresponding treatments applied (20). It is proposed that some acute inflammatory reactions after injection may be due to the stimulation of the natural inflammatory response (11), possibly by the release of reactive oxygen species and proteases from leukocytes, which are present in some PRP preparations (20).
Patel et al. (6) documented systemic adverse effects in patients receiving supra-patellar PRP injections, with the occurrence of immediate events such as syncope, dizziness, and tachycardia of short duration. The authors mentioned the use of CaCl2 as a possible contributing factor to these unfavorable events. In our study, some patients experienced mild pain in the site of injection between the first 24 hours, that was relieved with analgesics. None of the patients included in this study presented further complications after PRP treatment.
A low incidence but potentially catastrophic complication to consider when practicing intra-articular injections is the development of septic arthritis (21, 22). The correct use of aseptic conditions is mandatory for the preparation and administration of PRP, as well as contemplating the potential risk of complications in particular situations. Charalambous et al. evaluated the precautions taken during knee intra-articular corticosteroids injection, and reported a trend toward limited use of aseptic techniques (23).
Regarding PRP, there is one reported case of localized infection in the site of injection after its administration to treat an ulcerative lesion. The authors mention that the infection could have been induced by possible vascular damage during injection (24). In our study, there were no such complications, even in the group of patients with bleeding disorders, who underwent intra-articular injections of the knee, ankle, and elbow. It is of note that complications are more common among patients with hemophilia -as they present a higher incidence of septic arthritis- due to the pre-existing joint damage and the high rate of orthopedic procedures performed in these patients (25).
It is worth mentioning that PRP has been recognized to exhibit antimicrobial properties, as it is capable of inhibiting growth of different bacterial strains. This ability is due mainly to the presence of microbicidal molecules in platelets, that directly or indirectly inactivate pathogens, making PRP a formulation with the potential to prevent operative and postoperative infections (26-31). This property gives PRP additional advantages over conventional therapies. It is important to consider that our PRP preparation and administration protocol under sterile conditions considerably reduce the chances of contamination and further complications associated with it.
5.1. Conclusions
Platelet-rich plasma treatment is applicable to many different etiologies, involving both intra-articular and soft tissue procedures, with nil risk of infection when performed under controlled conditions. It is a minimally invasive procedure whose safety extends to patients with hemophilia, also in the presence of inhibitors and infectious diseases often associated with hemophilia treatment.