1. Introduction
Atopic dermatitis (AD) is a chronic, recurrent, inflammatory skin condition with a critical immunological component, defined by the presence of eczematous, pruritic lesions in specific locations (depending on age), also associated with patients with asthma and/or allergic rhinitis (1). Regarding clinical definition, Hanifin's and Rajka's major and minor criteria have been used to evaluate the severity of AD. Also, the scoring atopic dermatitis (SCORAD) index has been developed, allowing for better evaluation and standardized follow-up of patients (2) During the past three decades, there has been an increase in the prevalence of AD in the general population, especially in infants, and 60% of cases occur during the first year of life (3).
The pathophysiology of AD has been attributed to impairment of the epithelial barrier, which causes skin fragility and makes the skin more prone to erythematous reactions resulting from the biological response restoring skin homeostasis. Cornification, lipid secretion, and stimulation of innate defense are involved in this process. The association of fragile skin with the restorative response in patients with AD makes them less tolerant and more reactive to different stimuli (4).
The immunopathology of AD is complex, comprising humoral immunity disorders, for example, changes in IgE concentrations, cellular immunity (with type IV reaction), and other immune cells, such as decreased stimulation of lymphocytes by Langerhans cells. In cases of AD, intracellular levels of cyclic AMP are reduced, which is responsible for the consequent increase in histamine secretion, decrease in suppressor T lymphocytes, and increase in IgE levels, in addition to the presence of abnormalities in arachidonic acid metabolism. These factors contribute to inflammation persistence (5).
Total IgE levels are related to age, sex, ethnicity, family history of atopy, contact with allergens, and parasitosis. Serum IgE increases progressively with age until adolescence and begins to decrease in adulthood. In children, normal total IgE levels are low, and increased levels are important in diagnostic evaluation, especially when diagnosing allergic diseases, such as contact dermatitis (CD), asthma, and parasitosis (6).
Contact dermatitis is a differential diagnosis of AD, given that they are both eczematous, pruritic conditions that may affect diverse locations (7). There are two primary forms of CD, irritant and allergic. Irritant CD due to topical substances is triggered by agents that cause direct tissue damage without prior sensitization, whereas allergic CD results from sensitization to a substance that induces a cell-mediated type IV hypersensitivity reaction (8).
Advances have made it possible to realize that patients with chronic skin conditions show greater susceptibility to developing CD due to topical drugs as a result of the modified skin barrier (in the same manner as in patients with AD), and they are frequently exposed to these medications. We describe a patient with AD complicated by CD due to successive treatments implemented, who improved following the suspension of treatments and restoration of the skin barrier.
2. Case Presentation
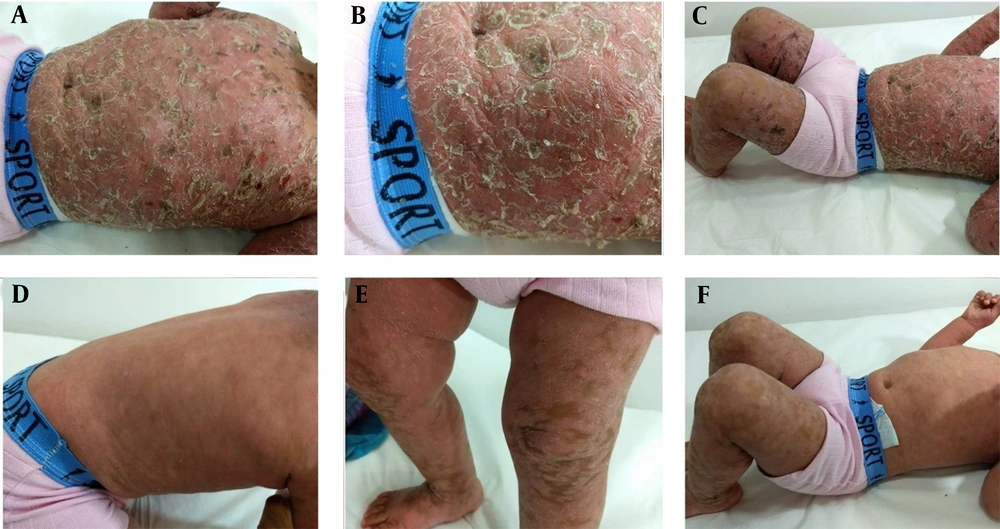
We report a two-year-old male patient, with a prior diagnosis of AD since the age of 10 months, with recurrent episodes of exacerbation and remission while using topical and/or oral medications. He had undergone numerous treatments, including topical corticotherapy, isolated and associated with an antifungal agent, corticosteroids, and oral antibiotic therapy. He progressed with important worsening of the lesions, irritability, intense pruritus, and movement limitation caused by severe skin impairment. His mother stated that he did not have systemic symptoms or previous allergies and had not previously had lesions similar to those in question. There was no family history of skin conditions. Upon physical examination, the patient's clinical picture was erythrodermic, with xerosis and desquamation in thick brownish scales, which adhered to the center and had raised edges without ectropion or eclabium (Figure 1). The diagnostic hypotheses were congenital ichthyosis, CD, and erythrodermic AD.
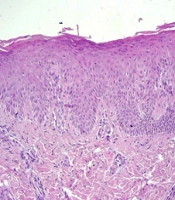
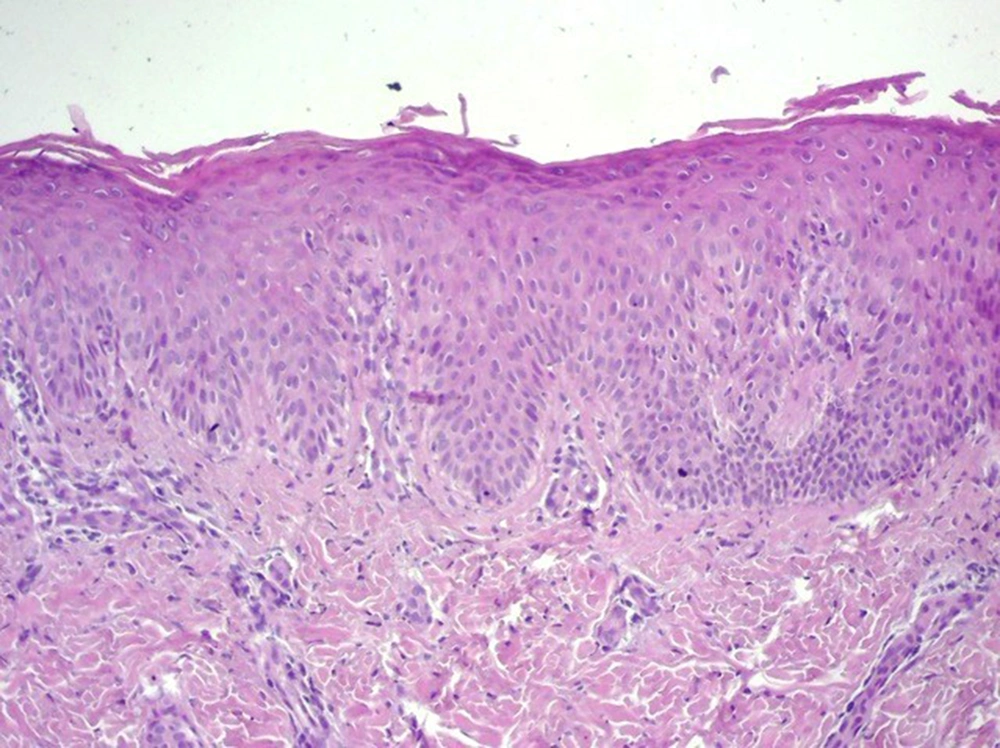
Therefore, all medications in use were discontinued; soap for restoring skin pH was prescribed, and a biopsy was conducted. Histological alterations revealed hyperkeratosis with parakeratosis, regular acanthosis of the epidermis, and an unaltered granular layer, which were compatible with a chronic inflammatory process (Figure 2). The patient improved significantly due only to the suspension of topical medications, thus inferring a condition of iatrogenic CD (Figure 1).
3. Discussion
Contact dermatitis due to topical medications can be iatrogenic or caused by a patient’s self-medication (8). Often associated with allergic CD, the causative agent may be the medication's active ingredient or one of its vehicles, including excipients, preservatives, antioxidants, emulsifiers, and fragrance components. The main drugs involved are local antibiotics, antiseptics, non-steroidal anti-inflammatory drugs, local anesthetics, and corticosteroids (9).
A retrospective study by Romita et al., from 2014 to 2016, sought to relate a greater predisposition to contact dermatitis in children affected by atopic dermatitis. In this study, 268 children under 14 with a history of eczematous dermatitis were evaluated, of whom 141 (52.6%) were affected, and 127 (47.4%) were unaffected by atopic dermatitis. All children were submitted to a patch test with the baseline SIDAPA Series standard to verify whether atopic children were more prone to allergic contact dermatitis and which substances were most frequently related to this disease. Based on this study, the prevalence of contact allergy in atopic children was comparable to that observed in non-atopic children. The most frequent causes of contact allergy were perfumes, with a significantly higher prevalence in atopic children (19.9%) than in non-atopic ones (11.8%; P < 0.05). Hence, it was inferred that the prolonged use of skin products, associated with the impairment of the skin barrier of atopic children, increases the risk of sensitization to the ingredients of these products (10).
In a cross-sectional study by Simonsen et al. (11), 100 children and adolescents aged 5 to 17 years diagnosed with atopic dermatitis were tested with a pediatric series of 31 allergens. Thirty percent of children had at least one positive patch test reaction, and 17% had at least one contact allergy relevant to their current skin symptoms. The risk of contact allergy was significantly correlated with the severity of atopic dermatitis. Metals and components of topical skin care products were the most frequent sensitizers. Mailhol et al. (12) conducted a study to assess the frequency of sensitization to the topical treatment of atopic dermatitis in children and determine the risk factors associated with skin sensitization. They systematically tested 641 children with atopic dermatitis tested with seven common topical treatment agents: Chlorhexidine, hexamidine, budesonide, tixocortol pivalate, bufexamac, sodium fusidate, and with the current emollient used by the child. The authors concluded that topical treatment of AD was associated with skin sensitization. Antiseptics and emollients represent the most frequent sensitizers and can be included in the standard series in children with AD when contact dermatitis is suspected. Risk factors associated with sensitization to topical AD treatment are AD severity, early onset of AD, and IgE-mediated sensitization.
The AD pathophysiology is represented by alterations in the stratum corneum, resulting from decreased levels of long-chain fatty acids, ceramides, and intracellular lipids; this contributes to greater transepithelial dehydration and increased permeability to allergens. The immune response is predominantly Th2 and Th22, stimulating the production of B lymphocytes and IgE by interleukins. On the other hand, the immune response to CD is characterized as type IVa, according to Gell and Coombs classification, which is a Th1 response, with the release of cytokines and activation of macrophages (13).
As known, AD begins with cutaneous alterations mediated by the Th2 response during the chronic phase, and the inflammatory process is described as a shift to the action of the Th1 response. Exposure and entry of allergens/irritants initiate a change in the upregulation of the Th2 response, reinforcing the effects of AD and creating interaction between the responses to AD and CD (14). Therefore, the correlation between the conditions must be evaluated because patients with AD have an incompetent skin barrier and may present sensitization to substances. It is advisable to carry out the patch test in patients with acute or chronic conditions, especially when treatment does not lead to resolution, indicating the possibility of the associated allergic CD where AD does not respond well to the treatment (15).
Corticosteroids are widely used to treat pathologies due to their anti-inflammatory and immunosuppressive nature; they can, however, be responsible for hypersensitivity reactions. The increased risk of contact allergy to corticosteroids is related to the disease duration and the duration of topical steroids. Sensitization results from the products of glucocorticosteroid degradation after their conversion in the epidermis, giving rise to the hapten known as glyoxal, which has a high capacity for allergic induction after making a combination with a protein (16). According to studies, contact allergies to glucocorticosteroids may affect 12.8% of patients with AD, 20% of patients with contact eczema, and 40% of patients with chronic leg ulcers (17).
Patients with chronic skin conditions and impaired skin barrier, when having CD due to medication use, demonstrate difficult etiological diagnosis, given that they often use various medications, whether on account of self-medication or erroneous medical prescription (18). When managing patients with AD, it is essential to use the lowest possible number of drugs and implement well-documented non-pharmacological measures to control the disease with less likelihood of iatrogenic effects, such as the ones described herein.