1. Background
Cupping therapy, one of the oldest documented medical techniques, has been used for several thousands of years in ancient countries, like China, Iran, Egypt, and Greece (1). Wet cupping could be used for treatment of several disorders, including skin, metabolic, and cardiovascular diseases (2). Avicenna, the famous Iranian physician practicing more than 1000 years ago, wrote a book named “The canon of Medicine”, which was a standard medical text in Europe and the Islamic word until the 17th century, in which he describes the details of different kinds of cupping therapy. He mentioned that cupping could manage over 37 kinds of diseases (3). In Europe, it was widely used from medieval times up to the 19th century (4). It is done using cups placed on desired spots on patient’s skin to cause hyperemia and homeostasis. There are 2 types of cupping practices, including dry cupping and wet cupping. Cupping is usually done using flame heating power to achieve suction inside the cups. In dry cupping, skin is pulled into the cup without bleeding (5). The second type is wet cupping or bleeding cupping (Hijamat). In this method, after suctioning the desired place by cupping, the practitioner makes small incisions using a triangle-ended needle to cause bleeding and again make suction using cupping. After emptying the cup, this step is repeated 2 or 3 times. It is believed that this method removes harmful blood lying beneath the skin surface (6). Some systemic review articles demonstrate significant therapeutic effects of wet cupping on skin disorders, including herpes zoster (2, 7). The current work focused on wet cupping and compared the differences between samples of cupping blood and venous blood. Cupping improves subcutaneous blood flow circulation and stimulates autonomous nervous. This method is used in different diseases, like pain, hypertension, headache, anxiety, and rheumatoid arthritis (8-12). Cupping therapy does not have any major side effects. It may cause minimal discomfort because of suction and skin cuts to the patient or feeling of slight light-headedness after cupping due to blood flow to the cupping region. Pregnant or menstruating females, cancer patients with metastasis, patients with bone fracture, and the site of DVT are contra-indicated (13).
Metabonomics is the study of low molecular weight components, which gives information about the function of the entire organism. It measures metabolites within cells, biofluids or tissues during a modification or stimulation. This technique is applied to study the effects of diet, toxins, drugs, stress, and a verity of diseases. Analytical tools for measurement include mass spectrometry (MS), 1H nuclear magnetic resonance (1HNMR), high performance liquid chromatography/mass spectrometry (HPLC/MS) and gas chromatography mass spectrometry (GC/MS) (14). The 1HNMR used in this study could measure all metabolites, simultaneously, and the sample that does not require pre-treatment can be recovered for further analysis (15). A preliminary study was carried on healthy males to compare the metabolomics difference on venous and cupping sera to compare their differences.
2. Methods
2.1. Ethics Statement
Ethics committee of Pasteur Institute of Iran approved the protocol (P/24/M/T940, dated: 25.3.2012 and IRCT ID: IRCT2014121620329N1).
This study was a controlled, clinical trial on 20 healthy males (mean age: 35), who attended the traditional medicine clinic of Maad Iranian for annual cupping, and voluntarily participated in the study. They were not alcoholics, addicted, and did not use specific medications.
2.2. Sample Collection and Preparation
After completing the informed consent and questionnaire, venous blood samples were collected in serum tubes before treatment. Then wet cupping was carried out as follows:
1- The area between the 2 scapulas, opposite the (T2 - T5) scapular spine was disinfected by rubbing alcohol. This is the standard area for wet cupping.
2- After placing the cup on the selected area, manual suction was used to exclude the air inside the cup. The cup was clanged to the skin for 3 to 5 minutes.
3- After removing the cup, multiple superficial incisions were made on the skin using sterile surgical blades.
4- Again the cup was placed on the skin, as mentioned above, for 3 to 5 minutes, to a point it was filled with capillary vessel blood.
5- Four milliliters of the collected blood was spilled in a tube for serum collection and metobonomics analysis.
6- The place was dressed using sterile dressing and a layer of honey, which facilitates wound healing (10).
2.3. Biochemical Testing
By centrifuging the blood samples at 3000 rpm for 15 minutes, sera was separated and used for metobonomics analysis and biochemical parameters, including uric acid, high density and low density lipoproteins (HDL, LDL), Triglyceride (TG), SGOT, and SGPT by Technicon RA1000. The serum was stored at -800 C until analysis.
2.4. 1H Nuclear Magnetic Resonance spectroscopy
This study used one dimensional 1HNMR spectra, Bruker DRX-400 NMR spectrometer operating at 500.13 MHZ at 298k. For weighting the free induction decay (FID), an exponential function with a 0.3 - HZ line broadening factor prior to fourier transformation (FT) was applied for each serum sample. To suppress water pre-saturation pulse sequence (D-90-t1-90-tm-90-acquired FID) with relaxation delays of 5 seconds and flip angle of 90, water signals and the broad protein resonances, a combination of pre-saturation and the Carr-Purcell-Meiboom-Gill (CPMG) 90-(t-180-tn-acquisition) (τ = 200, n = 100) pulse sequence (16). Phase and baseline correction were made for each spectrum. Spectral width was 8992.806 HZ and acquisition time was 0.911 seconds.
2.5. Data Reduction
The CPMG-NMR spectra were segmented to a region of 0.01 ppm in width using the Chenomix 6.4 software by the binning option on the software. After calculating the integrated area under each of these region’s curve (which are called “bins” or “buckets”), these values were used as variables. To eliminate variation in water suppression efficiency, the region of the spectrum with the water signal (δ 4.44 - 5.2) was removed from analysis for all the groups. After calculating the area for each segmented region, the integral values were shown in an intensity distribution and description of the whole spectrum with 1000 variables prior to PLS analysis. These data were normalized by setting it to the total region of each spectrum.
2.6. Chemometrics Analysis
2.6.1. Partial Least Square (PLS)
First orthogonal signal correction (OSC) filters with spectral data was set as the X matrix and the samples data sets were labeled as 0 for venous and 1 for cupping blood for the Y matrix (17). The PLS was performed with and without OSC and then the results were calculated with more than 95% confidence levels.
2.6.2. Orthogonal Signal Correction (OSC)
Filters of OSC were calibrated to remove unwanted variation from spectral data (18). Matrix X shows before laser data of 1HNMR samples and matrix Y as after laser 1HNMR data. The OSC subtracts from X; factors that account for the variance in X and are orthogonal to Y. To prevent poor predictive performance, it is important to avoid over fitting after OSC, therefore, precise determination of the number of removed OSC factors is very important. In this study only one factor was removed.
2.7. Detection of Metabolites
The link of NMR search for human metabolome database (HMDB) was applied to detect metabolites in each certain chemical shift. It is a freely available electronic database containing detailed information about metabolites found in the human body.
2.8. Metabolic Pathway Analysis
For this analysis, MetaboAnalyst 2.0 was used in pathways, in which the differentiating metabolites were analyzed (19). Using this database, this study used pathway-associated metabolite sets with enrichment analysis and the main pathways affected were detected.
3. Results
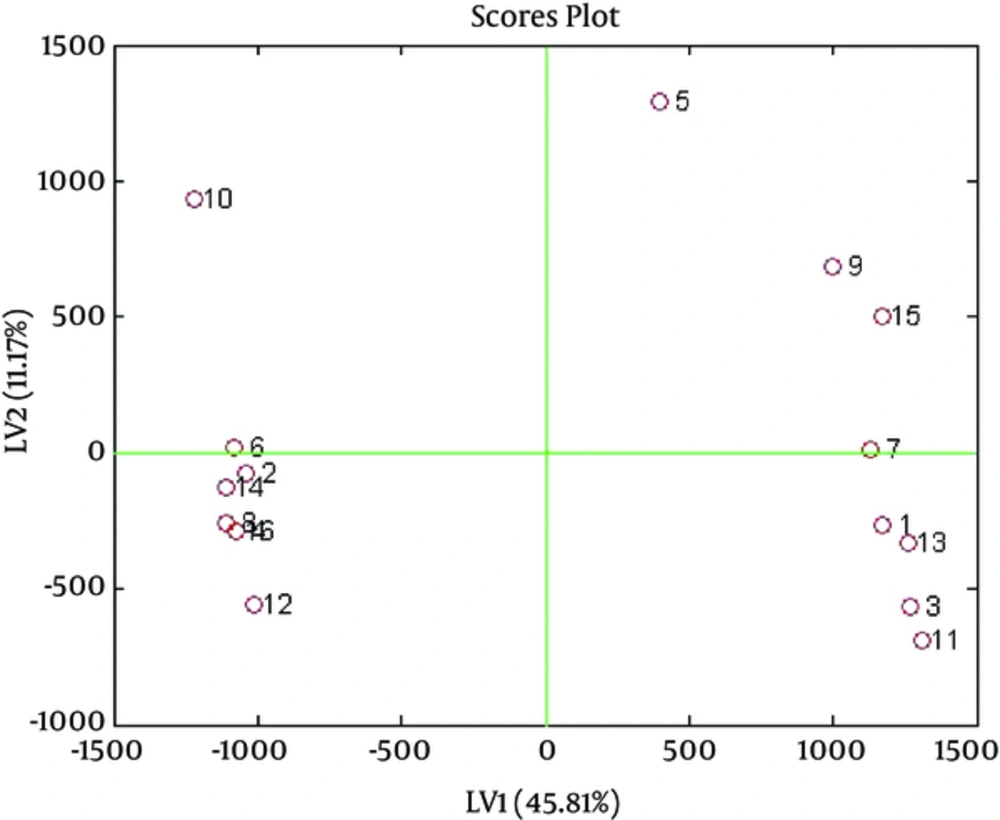
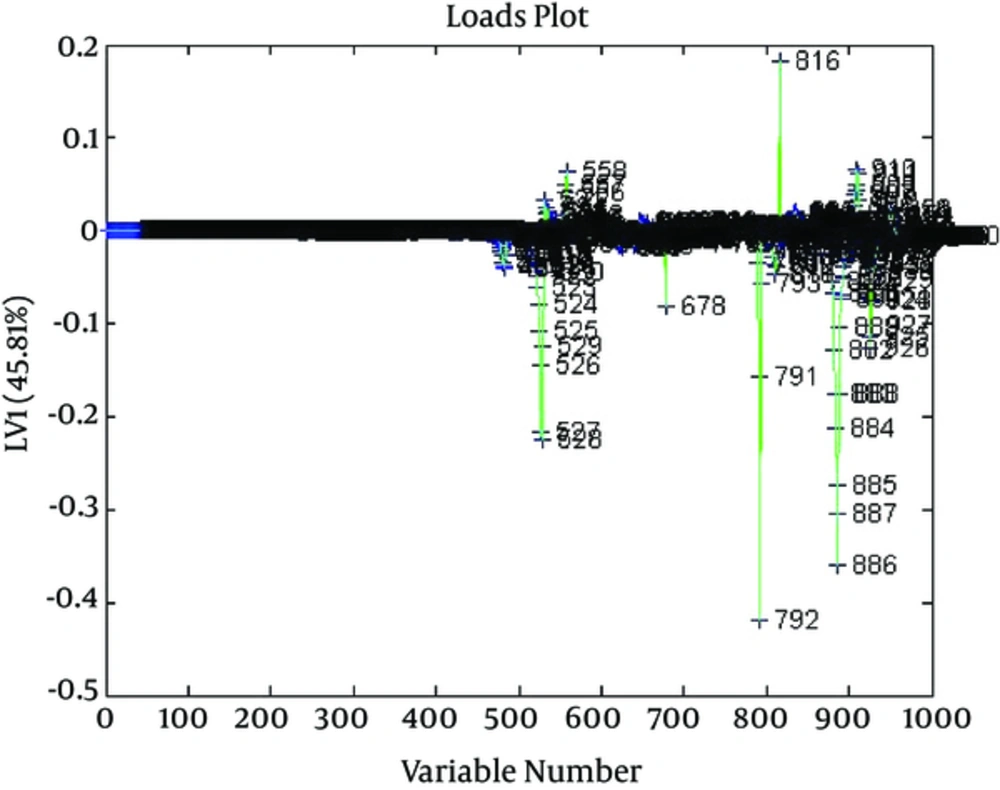
Analysis by 1HNMR: Results of PLS following OSC were satisfactory and the 2 groups (venous blood sample and cupping blood sample) were separated very well. Figure 2 shows score plot and Figure 3 shows biplot of OSC-PLS of these samples.
The chemical shifts were detected using the numbers of differentiating metabolites and the metabolites were recognized using HMDB testing, as indicated in Table 1.
| No. of Metabolite | Name of Metabolite | HDMB No. | Levels in Cupping Blood |
|---|---|---|---|
| 1 | S-Adenosyl-homocysteine | HMDB00939 | ↓ |
| 2 | Pyridoxamine | HMDB01431 | ↓ |
| 3 | Nicotinamideribotide | HMDB00229 | ↓ |
| 4 | Deoxyadenosine | HMDB00101 | ↓ |
| 5 | Cortisone | HMDB02802 | ↓ |
| 6 | L-Arginine | HMDB00517 | ↑ |
| 7 | D-Glucose | HMDB00122 | ↓ |
| 8 | 7-Dehydrocholesterol | HMDB00032 | ↓ |
| 9 | 1-2Hydroxyglutatic acid | HMDB00694 | ↓ |
| 10 | 3-Hydroxybutyric acid | HMDB00357 | ↓ |
| 11 | CE (16.0) | HMDB00885 | ↓ |
| 12 | Taurocholic acid | HMDB00036 | ↓ |
| 13 | CE (16.1 (9Z) | HMDB00658 | ↓ |
| 14 | Epiandrosterone | HMDB00365 | ↓ |
| 15 | 7-Ketocholesterol | HMDB00501 | ↓ |
| 16 | Aldosterone | HMDB00037 | ↑ |
| 17 | Cholesterol | HMDB00067 | ↑ |
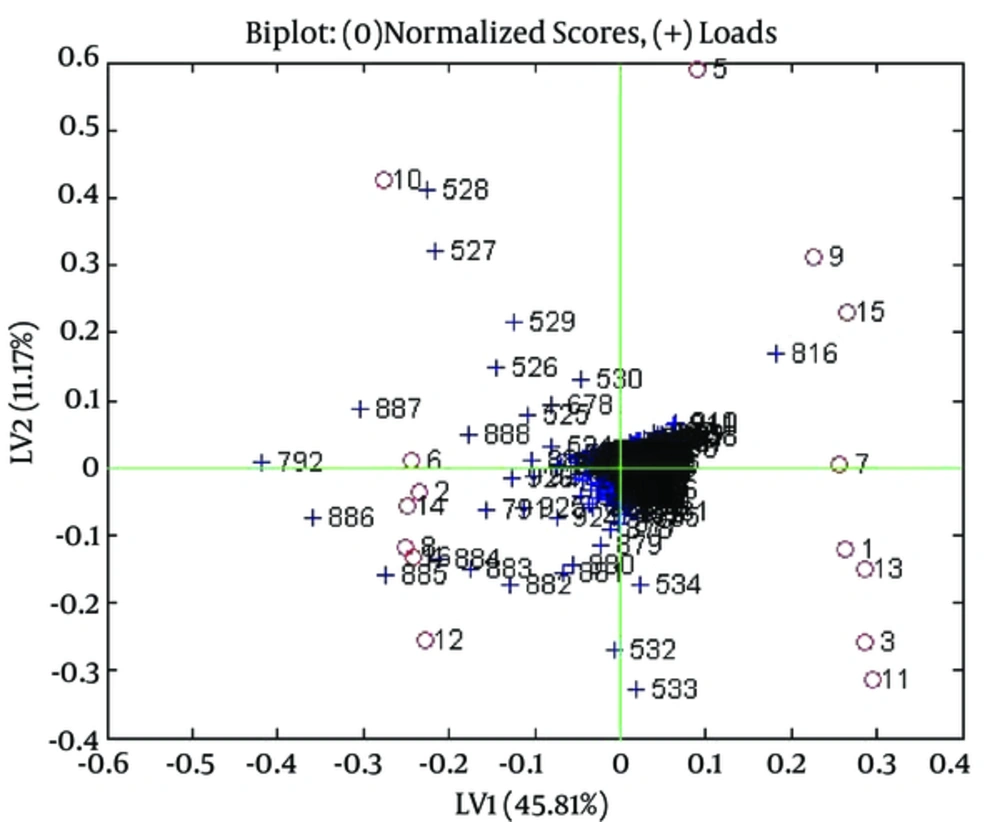
The samples were separated on the basis of their differentiating metabolites. Figure 2 is the score plot and Figure 4 is the loading plot.
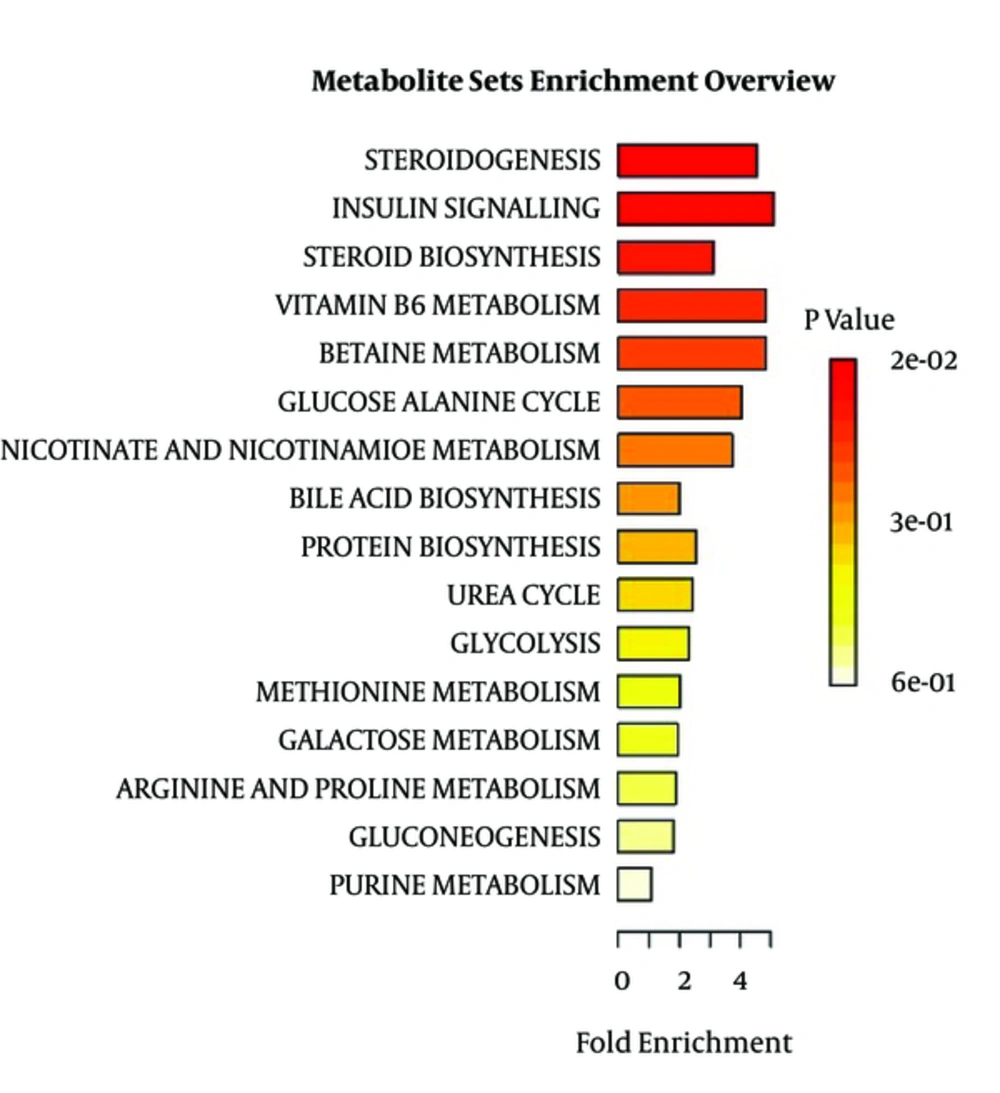
The list of metabolites was used in enrichment analysis and the main pathways affected could be seen in Figure 5.
The pathway analysis in Table 2 shows the main pathways affected in descending order. Number of hits show the number of metabolites, which were changed in the cycle. Raw P is the P value of the cycle.
| Metabolites | Total | Expected | Hits | Raw P |
|---|---|---|---|---|
| Steroidogenesis | 32 | 0.66 | 3 | 2.49E-02 |
| Insulin signaling | 19 | 0.39 | 2 | 5.56E-02 |
| Steroid biosynthesis | 31 | 0.64 | 2 | 1.31E-01 |
| Vitamin B6 metabolism | 10 | 0.21 | 1 | 1.89E-01 |
| Betaine metabolism | 10 | 0.21 | 1 | 1.89E-01 |
| Glucose-alanine cycle | 12 | 0.25 | 1 | 2.22E-01 |
| Nicotinated and nicotinamide metabolism | 13 | 0.27 | 1 | 2.39E-01 |
| Bile acid biosynthesis | 49 | 1.01 | 2 | 2.68E-01 |
| Protein biosynthesis | 19 | 0.39 | 1 | 3.30E-01 |
| Urea cycle | 20 | 0.41 | 1 | 3.44E-01 |
| Glycolysis | 21 | 0.43 | 1 | 3.58E-01 |
| Methonine metabolism | 24 | 0.49 | 1 | 3.98E-01 |
| Galactose metabolism | 25 | 0.52 | 1 | 4.10E-01 |
| Arginine and proline metabolism | 26 | 0.54 | 1 | 4.23E-01 |
| Gluconeogenesis | 27 | 0.56 | 1 | 4.35E-01 |
| Purine metabolism | 45 | 0.93 | 1 | 6.18E-01 |
4. Discussion
Despite the progress of modern medicine in diagnosis and management of many diseases, it is unable to control and prevent many cases of chronic disease. Several studies have shown the efficacy of alternative and complementary medicine, especially in the management of chronic disease and cancers or skin disease, including acne, hair loss, and urticaria (20-22). Although cupping therapy has been used for thousands of years in traditional medicine, clinical studies on cupping therapy have only been performed during the past 10 years. Therapists, who use wet cupping, believe that some harmful substances are present in the blood and cause different diseases and wet cupping removes them and may create a balance in the body (23, 24), however several researchers do not believe in differences between cupping blood and venous blood samples. The present study has for the first time used a powerful analytical method to determine differences between these 2 blood samples and showed that cupping therapy removes harmful substances from the blood. There are about 17 metabolites differentiating the 2 kinds of sera. The pathway impact as checked by Metaboanalyst has shown that about 17 pathways differ between the two, of which the first 7 pathways are very significant.
Cupping blood seems to differ with venous blood in the following metabolites and metabolic cycles: cholesterol, cortisone, and aldosterone effecting stereodiognesis insulin signaling due to presence of D-glucose and E (16: 0), steroid hormone biosynthesis involving 7-dehydrocholesterol, and cholesterol. Vitamin B6 metabolism is affected by pyridoxamine. Betainemetabolism is also seen to have differed by s-adenosylhomocysteine. Glucose alanine cycle is detected due to the presence of D-glucose. Nicotinamideribotide has been reported to participate in nicotinate and nicotinamide metabolism. Pyridoxamine, nicotinamide, and aldestrone are involved in some skin disorders, including hair loss, hirsutism, and acne. Studies show that elevated level of aldestrone may cause hirsutism signs and hair loss (25, 26). The results show that cupping blood contains significantly higher amounts of aldestrone and lower amounts of pyridoxamine and nicotinamide in comparison with venous blood. Bile aid bio synthesis due to taurocholate andcholesterol, L-arginine is seen in protein biosynthesis and urea cycle and D-glucose in glycolysis. These are metabolites and metabolic cycles, which differed significantly between the 2 groups.
El-Domyati et al. studied the effects of wet cupping on the treatment of chronic idiopathic urticaria (CIU), dermatitis, vitiligo, psoriasis vulgaris, and acne vulgaris. Their results showed significant decrease of serum IL-2 and IgE level and significant increase of serum C3 level. They concluded that it could be considered as an effective alternative therapy for acne vulgaris and CIU (27).
Several studies demonstrate the effects of wet cupping in the treatment of acne vulgaris (28, 29).
The presence of cortisone in metabolites of significant difference in the current study is very interesting. As wet cupping has analgesic effects like acupuncture and acupressure, Rozenfeld and Kalichman suggested a similar mechanism for pain relief that could extract the release of serotonin, morphine-like substances (endorphins) or cortisol, which may lead to pain relief and change in the physiological state (30). It could be claimed that the effect of acupuncture and acupressure is by activating or stimulating: 1. Dilation and vasoconstriction; 2. Release of neurotransmitters; 3. Secretion of enkephalins; 4. The immune system, and 5. The pain gates (by affecting on gate control in the central nervous system that interpret pain sensation). It seems that dry cupping may apparently activate the same pathways. In relation to the immune synthesis, protein biosynthesis is an important cycle detected by this study. Cupping may affect the immune system in 2 ways: by irritating the immune system, which causes local inflammation, and subsequently activates the complement system, and increasing the level of interferon and tumor necrotizing factor (TNF); or by increasing the lymph flow, in which protein biosynthesis plays an important role. It is also important to mention that glucose, as one of the differentiating metabolites, was seen in glycolysis and glucose alanine cycles. Earlier studies have also shown that cupping is able to increase the lactate/pyruvate ratio after 160 minutes, indicating an anaerobic metabolism in the surrounding tissue with immediate increase of pressure pain thresholds in various areas. Alanine is converted to pyruvate and then lactate in the muscles, and in the liver is converted back to glucose, after entering the urea cycle, alanine is shuttled to the liver where the nitrogen enters the urea cycle and the pyruvate is used to make glucose (31).
In another study, venous and wet cupping blood samples were collected at the same time. Measurement of serum malondialdehyde levels, nitric oxide, and activity of superoxide dismutase and myeloperoxidase were taken and it was seen that wet cupping blood had lower activity of superoxide dismutase, higher activity of myeloperoxidase, and levels of nitricoxide and malondialdehyde compared to the venous blood. It seems that wet cupping decreases oxidative stress by removing oxidants. It is very interesting that this study found the presence of adenylhomocysteine as a differing metabolite, which participates in nicotine and nicotineamide metabolism and is classified as vitamin B3 and participates in anti-oxidation activity (32).
Cao et al. reported in a literature review that cupping therapy appears to be improved in China, and studies show its benefit on acne, pain, and other conditions. No serious adverse effects were reported in the reviewed studies (7). In a systemic review of randomized control trials they reported that wet cupping could be effective in treatment of herpes zoster (2). Albedah et al., in a review reported the efficacy of wet cupping in treatment of acne, herpes zoster, and headache (6). Farhadi et al. showed the efficacy of wet cupping therapy in treatment of non-specific low back pain in Iranian patients (11). Lubtke et al. reported that brachialgia paraesthetica nocturnal could be relieved by wet cupping, however this trial lacked adequate and suitability of blinded placebo treatment (33). Lee et al. evaluated the effect of wet cupping on hypertension and stroke in 2 systemic review literatures. They reported that one step of wet cupping could reduce acute hypertension (9, 34). In a systemic review done by Albedahet al. (6), they reported some suggestive evidence for the effectiveness of cupping in pain management, but for stroke rehabilitation, they did not find enough evidence for the effectiveness of cupping therapy. Ahmed et al. reported that cupping combined with conventional therapy, improved pain, tenderness, and swelling in patients with rheumatoid arthritis and had significant immune modulatory effects (10). It would be interesting to see which of these pathways maybe involved in alleviating the pain whether it is steroid hormone biosynthesis or some other pathway. Michalsen et al. evaluated the effect of cupping therapy on carpal tunnel syndrome and reported that this method maybe effective in pain relief and other related symptoms (35). Ahmed et al. evaluated the effect of cupping therapy on blood parameters in patients with rheumatoid arthritis. Their study showed significant reduction in laboratory markers of disease activity and modulation of immune system, especially natural killer cells and adaptive cellular immune response SIL-2R (10). Niasari et al. evaluated the effect of wet cupping on serum lipid concentration. They suggested that wet cupping may affect the decrease in low density lipoproteins and may consequently prevent atherosclerosis (24). It is worthy to note that the differentiating metabolites included cholesterol and hydroxyl butyrate, which are involved in bile acid biosynthesis and synthesis and degradation of ketone bodies. Bilal et al. compared the blood samples obtained through the cupping method with venous blood samples and reported a significant difference in almost all tested parameters between cupping samples and venous blood (23).
4.1. Conclusion
This study showed that wet cupping blood samples have a significant difference in nature compared to venous blood samples. The study found 17 metabolites and 16 metabolic cycles differing between them and 8 of them were the most significant, including cholesterol, cortisone and aldosterone effecting steroid hormone biosynthesis, primary bile acid biosynthesis, involving cholesterol and taurocholic acid, synthesis and degradation of ketone bodies with 3-Hydroxybutyric acid, L-Arginine in D-Arginine and D-ornithine metabolism pathway, Taurine and hypotaurine metabolism affected by taurocholic acid, D-glucose, responsible for glycogenesis and gluconeogenesis and pyridoxamine participates in Vitamin B6 metabolism. The current study suggests wet cupping as an effective treatment in treating a variety of medical diseases, including diabetes, lipid disorders, cardiovascular disease, and immune system disease and several skin disorders including hair loss, hirsutism, acne, urticarial, and psoriasis.