1. Background
Since neural crest-derived melanocytes, melanin-producing cells control human skin color, it is important for skin and stem cell science to make clear the mechanism of growth and melanogenesis of melanocytes in humans. Human melanocytes are mainly located in the epidermis and hair follicles (1). Melanocytes are thought to be essential in skin color expression and protection of the skin against harmful stimulants such as ultraviolet radiations (UVR) (1). L-tyrosine is a starting material of melanin biosynthesis (2, 3). Three kinds of enzymes, tyrosinase, tyrosinase-related protein-1, and tyrosinase-related protein-2, mainly regulate melanin biosynthesis (2, 3).
Melanocytes differentiate from melanoblasts that derive from the neural crest in the skin of the embryo (4, 5). Melanoblasts enter the epidermis from the dermis at mid-gestational age and differentiate into melanocytes. On postnatal days, melanocytes in the epidermis move to hair follicles and complete the hair bulb with keratinocytes. Hair bulb melanocytes secrete melanosomes with full melanin depositions to keratinocytes and finally complete pigmented hair (6, 7).
Melanin biosynthesis occurs in pigment granules named melanosomes. Melanosome maturation is divided into 4 stages: Unpigmented melanosomes into stages I (initiation of intraluminal matrix formation) and II (completion of the intraluminal matrix), and melanized melanosomes into stages III (initiation of melanin depositions on intraluminal matrix) and IV (mature melanosome). Human melanocytes possess all stages of melanosomes, while melanoblasts possess only stage I and II melanosomes without tyrosinase activity (1, 2, 6, 7).
In mice, melanocytes are located in the dermis in addition to the epidermis and hair follicles. However, in normal human skin, no melanocytes are located in the dermis (1). Moreover, the human epidermis is very different from that of mice. Namely, the human inter-follicular epidermis is wavy, which is different from that of the murine flat inter-follicular epidermis. It derives from the fact that the human inter-follicular epidermis is rich in rete ridges (1, 6, 7). The human rete ridge epidermis possesses numerous melanocytes to prevent harmful UVR, but in adult mice, melanocytes are not present in the epidermis; thus, melanin present in hair follicles can prevent UVR (1, 6, 7).
The growth and melanization of human melanocytes are controlled by numerous growth factors and cytokines released from keratinocytes (8) and fibroblasts (9, 10). However, adequate attention has not been paid to the factors derived from dermal fibers, such as elastin fibers. Elastin fibers are involved in regulating the elasticity and strength of skin in cooperation with collagen fibers. Elastin fibers consist of 2 elements, microfibrils and matrix elastin. Fibrillins and microfibril-associated glycoprotein are major constituents of microfibrils (11). Elastin is synthesized and released from fibroblasts as a linear polypeptide known as tropoelastin. Elastin has a unique sequence, valine-glycine-valine-alanine-proline-glycine (VGVAPG): A hexapeptide that is repeated multiple times in human elastin molecules. Tropoelastin is released extracellularly and greatly cross-linked by lysyl oxidase (11).
Chang et al. (12) observed that during the development of C57BL/6J mouse skin, the development of epidermal melanoblasts/melanocytes is well correlated with the increased expression of the elastin-binding protein. Moreover, elastin-derived peptides (VGVAPG) directly stimulated the differentiation of melanocytes, dendritogenesis, and melanosome maturation in the cell culture system (12). These results suggest that in mice, the interaction between elastin molecules and elastin-binding proteins of melanoblasts/melanocytes is important for the development of melanocytes.
Ferrous ferric chloride (FFC; Akatsuka Corp, Mie, Japan) is known to regulate the oxidation and reduction states within the cells. Ferrous ferric chloride promoted the growth and function of murine (13) and human (14) skin cells, including keratinocytes, fibroblasts, and melanocytes in vitro, suggesting that FFC can regulate the normal function of human skin in situ.
Given these circumstances, we started to study whether the combined treatment of elastin peptides and FFC can promote the growth and melanization of melanoblasts and melanocytes in normal human skin. It is important for skin and stem cell science to understand the control mechanism of human skin homeostasis by melanocytes.
2. Methods
Normal skin sites (penis, buttock, scalp, neck, and wrist) from 6 Japanese volunteers (3 females and 3 males) aged 20 to 76 years old were skin samples (Table 1). Before starting the study, we explained the treatment and sampling methods and received informed consent from each patient. A lotion (FFC Venus Gelee, Akatsuka, Mie, Japan) containing elastin peptides (9 mg/mL) and FFC (0.001 ng/mL), as well as another lotion (FFC Super Essence, Akatsuka) containing FFC alone, was fully applied to their skin (2 cm in diameter) twice a day for 1 to 3 months from January to May 2019. Three punch biopsies (0.6 or 1.0 mm in diameter, 1.5 mm deep) per each skin site were taken by an electric micro drill at 90 g (SIRIUS NT MICRO, Gerlach Sirius Nt Micro, Eduard Gerlach GmbH, Luebeck, Germany) (15). The diameter of the sample (circle) size was calculated using a caliper, and the area of the circle determined was filled with a marker. Then, we took the skin sample using an electric cylinder needle of 0.6 or 1.0 mm in diameter. The needle was pushed on the marked area. We determined to include the accurate biopsy punch size for research and to exclude inaccurate size using a caliper. A total of 6 volunteers participated in this study (3 females and 3 males).
| No. | Gender | Age | Skin Site | 0 Months | 1 Month | 3 Months | |||
|---|---|---|---|---|---|---|---|---|---|
| Mc | Mb | Mc | Mb | Mc | Mb | ||||
| (A) FFC | |||||||||
| 1 | Male | 38 | Penis | 139.2 | 90.5 | - | - | 126.8 | 102.5 |
| 2 | Female | 20 | Buttock | 121.0 | 104.8 | - | - | 120.5 | 108.5 |
| 3 | Female | 45 | Scalp | 87.5 | 90.0 | - | - | 91.6 | 71.1 |
| Average | 115.9 ± 12.4 | 95.1 ± 4.0 | 113.0 ± 8.8 | 94.0 ± 9.5 | |||||
| (B) Elastin + FFC | |||||||||
| 4 | Female | 72 | Neck | 130.7 | 109.9 | 133.4 | 133.3 | 195.0 | 124.2 |
| 5 | Male | 76 | Scalp | 20.2 | 102.6 | 96.1 | 153.3 | 102.4 | 202.4 |
| 6 | Male | 56 | Wrist | 118.3 | 112.0 | 250.6 | 110.7 | 203.7 | 103.9 |
| Average | 117.2 ± 6.6 b | 106.0 ± 4.1 | 179.1 ± 29.6 | 115.5 ± 7.5 | 200.4 ± 2.2 b | 112.8 ± 4.9 | |||
Abbreviations: FFC, ferrous ferric chloride; Mc, melanocytes; Mb, melanoblasts
a Normal human skin was applied with lotions containing ferrous ferric chloride (A) or elastin peptides + ferrous ferric chloride (B). One and 3 months after the treatment, as well as before the treatment (0 months), 3 biopsies were prepared from the treated skin. They were then subjected to histochemistry, and the number of melanocytes and melanoblasts/0.1 mm2 were scored. Data are averages of 3 samples, and the standard errors of the mean are shown.
b A significant difference was observed by a statistical analysis using the t-test in the number of melanocytes between the control (0 months) and 3 months after the treatment (P < 0.001).
The methods of skin sampling and dopa reaction were reported previously (15, 16). Also, the method of combined dopa-premelanin reaction was reported previously (15, 16). The dopa reaction reveals melanocytes, and the dopa-premelanin reaction reveals melanocytes and melanoblasts (15, 17). The counting method of the number of melanocytes and the number of melanoblasts plus melanocytes in the epidermis was reported previously (15-17).
For elastin staining, deparaffinized sections were first treated with HBs antigen oxidizing solution (Muto Pure Chemicals Co, LTD, Tokyo, Japan) for 10 minutes at room temperature (RT). Then, the specimens were treated with 2% oxalic acid solution (Muto) for 30 seconds. After treating the specimens with 95% ethanol for 2 minutes, they were treated with Victoria blue staining solution (Muto) for 18 - 24 hours at RT. After treating the specimens with 70% ethanol to differentiate Victoria blue for 20 minutes, they were treated with nuclear fast red staining solution (Muto) for 20 minutes at RT.
The statistical analysis of the differences in melanocytes and melanoblasts between all groups was performed by the Student’s t-test (2-tailed).
3. Results
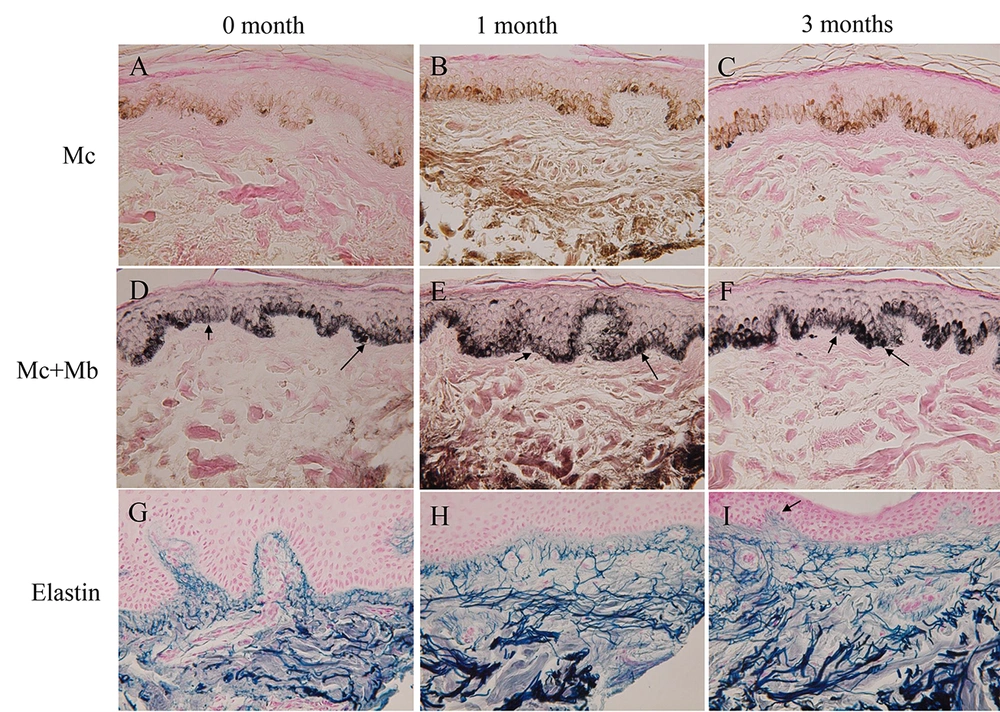
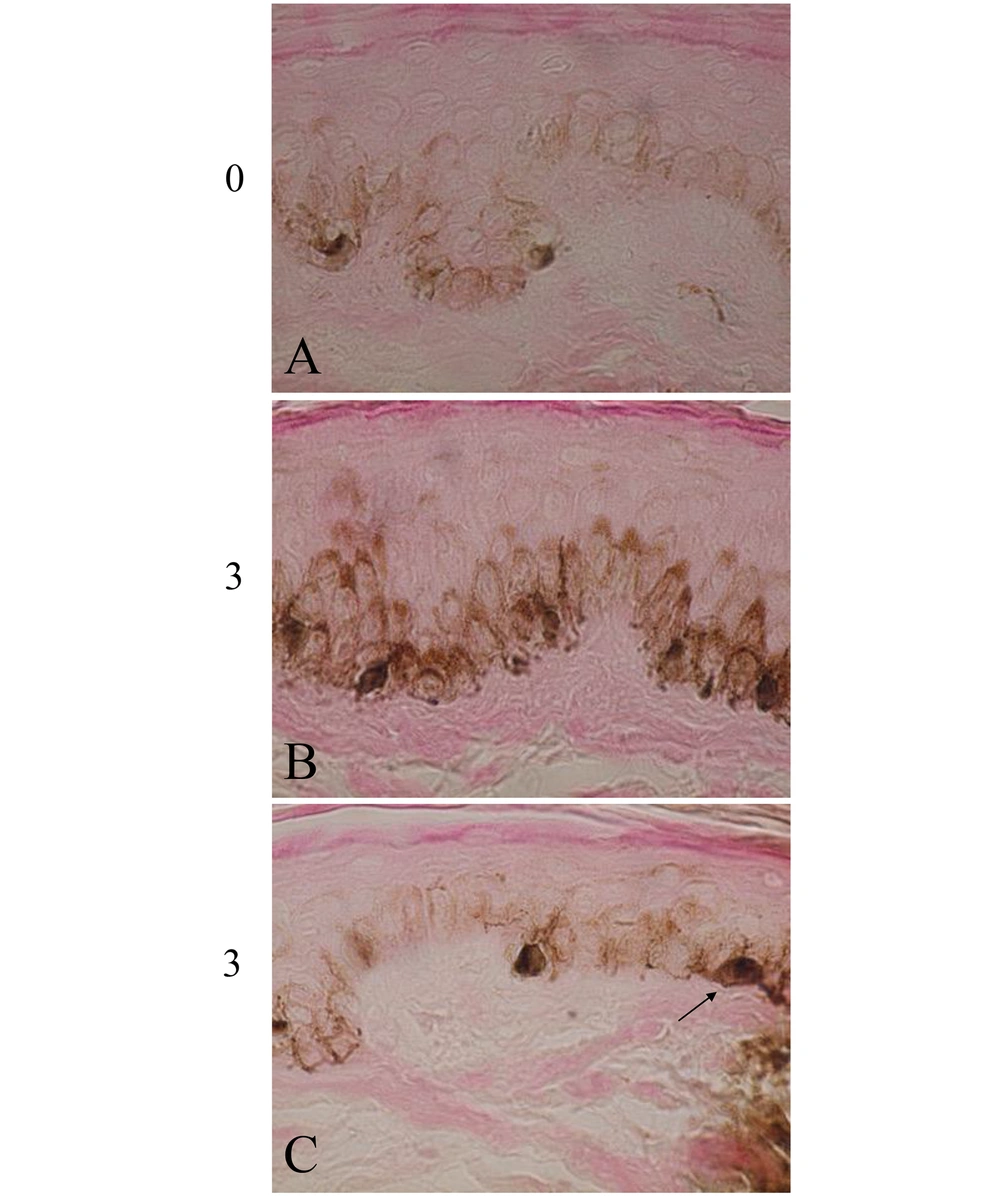
The skin color was gradually darkened in the 3 volunteers (Table 1, group B) treated with both elastin peptides and FFC, but not with FFC alone (Table 1, group A). Figure 1A shows the scalp skin of volunteer No. 5. Many dopa-positive melanocytes with dendrites were observed in the epidermis of the rete ridge and inter-rete ridge. Figure 1D shows the dopa-premelanin-positive cells. Melanocytes were darkly stained by this reaction, while melanoblasts were lightly stained. One month after the treatment, dopa-positive cells (Figure 1B) and dopa-premelanin-positive cells (Figure 1E) increased. Three months after the treatment, these cells further increased (Figure 1C and F). However, the increases in these cells were not observed in the skin treated with FFC alone (Table 1, group A). Higher magnification of the skin 3 months after the treatment revealed increased dopa-melanin depositions, dendritogenesis, and melanosome maturation in melanocytes, as well as increased epidermal melanin pigmentations (Figure 2B) compared to the control (the skin before the treatment; Figure 2A). In some cases, mitotic divisions of melanocytes were observed (Figure 2C, arrow). However, no mitotic divisions of melanoblasts were observed. In the other 2 volunteers (No. 4 and 6), similar tendencies were observed.
Histochemical sections of volunteer No. 5. The dopa reaction of the control skin (A) revealed many melanocytes with dendrites, and the combined dopa-premelanin reaction (D) revealed melanocytes and melanoblasts. Melanoblasts (D, short arrow) were lightly stained, while melanocytes (D, long arrow) were darkly stained. One month after the treatment, dopa-positive cells and dopa-premelanin-positive cells tended to increase. Three months after the treatment, they tended to increase further. Elastin fibers gradually increased 1 (H) and 3 (I) months after the treatment compared to the control (G). Elastin fibers became thicker and denser. Some of the elastin fibers penetrated the epidermis 3 months after the treatment (I, arrow). Magnification × 400.
Higher magnification photographs of the skin of volunteer No. 5. The dopa-melanin depositions in melanocytes and epidermal melanin pigmentations greatly increased 3 months (3) after the treatment (B) compared with the skin before the treatment (0, A). In some cases, mitotic divisions of melanocytes were observed 3 months (3) after the treatment (C). Magnification × 1000.
The average of melanocyte populations/0.1 mm2 from 3 volunteers treated with elastin + FFC (Table 1, group B) linearly increased after the treatment, but not in FFC alone (Table 1, group A). The difference in the melanocyte and melanoblast populations of FFC alone before and after 3 months was not statistically significant (both melanocyte and melanoblast: P > 0.5). Although the difference in the melanocyte populations of the treatment (elastin + FFC) before and after 1 month was not statistically significant (0.1 < P < 0.2), the difference after 3 months was statistically significant (P < 0.001). However, the averages of melanoblast populations/0.1 mm2 failed to increase both 1 and 3 months after the treatment (1 month: 0.3 < P < 0.4; 3 months: P > 0.5).
Elastin fibers also increased after the treatment with elastin peptides and FFC (Figure 1G, H, and I). Moreover, elastin fibers became thicker and denser. The combined treatment of elastin peptides and FFC seems to stimulate elastin fiber formation. However, these increases in elastin fibers were not observed in the skin treated with FFC alone. In the skin treated with elastin peptides and FFC, some of the elastin fibers penetrated the epidermis (Figure 1I, arrow), suggesting the possibility that elastin fibers interact with epidermal melanoblasts/melanocytes there. In the other 2 volunteers treated with both elastic peptides and FFC, similar results were also obtained.
4. Discussion
The present study demonstrated that the melanocyte population (but not the melanoblast population) increased after the application of the lotion, including both elastin peptides and FFC. Mitotic divisions of melanocytes (but not melanoblasts) were observed in the skin after the treatment. Furthermore, the depositions of dopa-melanin markedly increased, indicating that the activity of tyrosinase was increased by elastin peptides in the presence of FFC. The stimulation of dendritogenesis observed in the present study suggests that elastin peptides in the presence of FFC can stimulate melanosome formation and melanosome transport from cytoplasm to dendrites. Moreover, the stimulation of epidermal melanin pigmentation observed in this study suggests that elastin peptides in the presence of FFC can stimulate melanosome transport from melanocytes to keratinocytes (1). An increase in the skin color observed in this study is well correlated with an increase in epidermal melanin pigmentation observed histochemically. Taken together, elastin peptides in the presence of FFC seem to stimulate the growth of melanocytes, melanogenesis, melanosome formation, dendritogenesis, and melanosome transport from melanocytes to keratinocytes.
According to the results, the melanoblast population failed to be increased by elastin peptides in the presence of FFC, and no mitotic divisions of melanoblasts were observed, suggesting that the growth of melanoblast is not stimulated by this treatment. It has been reported that in the culture system, the growth of human melanoblasts is stimulated by FFC (13, 14). Although the reason is not clear why melanoblasts are not induced to proliferate, it might be possible that the combined treatment of elastin peptides and FFC may affect melanocyte proliferation and differentiation more easily than melanoblast proliferation. Indeed, in this study, dopa-reactivity and dendritogenesis of melanocytes, as well as epidermal melanin pigmentation, increased immediately after the treatment. The other reason may be derived from the experimental system, namely, the in vivo histochemical system in this study vs the in vitro culture system in the previous study (13, 14).
It has been reported that dermal melanocytes are intimately connected with elastin fibers (18). Chang et al. (12) demonstrated that the melanoblasts of normal embryonic mice expressed the elastin binding protein and unique hexapeptide (VGVAPG) derived from elastin molecule stimulated melanin synthesis and dendritogenesis of melanocytes, suggesting that the interaction between elastin and elastin binding protein-expressing melanoblasts is started early in the development, and elastin peptides can stimulate the differentiation of melanocytes. In the present study, an increase in the melanocyte population was well correlated with an increase in elastin fibers, suggesting that the interaction between epidermal melanocytes and dermal elastin fibers is important for melanocyte growth and development. Indeed, some of the elastin fibers were observed to penetrate the epidermis in the present study, suggesting that the direct connection between dermal elastin fibers and epidermal melanoblasts/melanocytes is performed even in normal human skin.
Cutaneous photoaging induced by chronic UVB irradiation is characterized by disorganized and non-functional depositions of elastin fibers (19). In the photoaged skin, elastin fibers generally decrease in density (20), and melanocytes decrease in number (21). However, the present results revealed for the first time that the lotion containing elastin peptides and FFC increased the number of melanocytes and the density of elastin fibers even in aged volunteers (72 and 76 years old). In mice, UV-induced skin photoaging was prevented by bovine elastin peptides, and these peptides inhibited the apoptosis of fibroblasts (22). Taken together, these results suggest that elastin peptides possess great potential to control photoaging of the skin in humans and animals.
Tian et al. (23) demonstrated that elastin peptides promoted tyrosinase activity, melanin content, and messenger RNA (mRNA) levels of endothelin receptor B and c-kit of A375 human melanoma cells in culture. Endothelin and c-kit are melanocyte growth factors (1). Consistent with their results, the present study indicated that elastin peptides in the presence of FFC increased dopa-melanin depositions (tyrosinase activity and melanin content), epidermal melanin pigmentation (melanosome transport to keratinocytes), and melanocyte growth. All these results suggest that elastin peptides can serve as cosmetic materials for treating skin hypopigmentation. On the other hand, Inoue et al. (24) reported that water-soluble pig aorta-elastin treated with hot alkali inhibited tyrosinase activity using reconstructed human epidermis (including melanocytes) in 3D culture. These studies suggest that the biphasic effects of elastin peptides on melanogenesis may be due to the differences in the kinds and concentrations of elastin peptides.
4.1. Conclusions
The combined treatment of elastin peptides and FFC is one of the effective treatments to activate melanocyte growth and melanization, followed by increased skin color. Thus, elastin peptides and FFC are thought to be useful molecules indispensable for maintaining human skin homeostasis and can be expected to serve as useful cosmetic materials for restoring skin hypopigmentation.